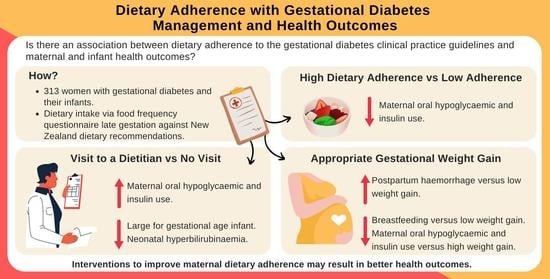
Adherence to Clinical Practice Guideline Recommendations in Women with Gestational Diabetes and Associations with Maternal and Infant Health—A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, S.T.; Hofer, O.J.; Harding, J.E.; Wall, C.R.; Crowther, C.A. Dietary Recommendations for Women with Gestational Diabetes Mellitus: A Systematic Review of Clinical Practice Guidelines. Nutr. Rev. 2021, 79, 988–1021. [Google Scholar] [CrossRef] [PubMed]
- Neiger, R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; DiMatteo, M.R. The Challenge of Patient Adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar] [PubMed]
- Mustafa, S.; Harding, J.; Wall, C.; Crowther, C. Sociodemographic Factors Associated with Adherence to Dietary Guidelines in Women with Gestational Diabetes: A Cohort Study. Nutrients 2021, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Lash, K.A.; Garcia, L.; Salazar-Laso, X.; Chahine, K.; Hotra, J.; Blackwell, S.C.; Refuerzo, J.S. Medication Adherence in Women with Gestational Diabetes and Its Effect on Pregnancy Outcomes. Am. J. Obstet. Gynecol. 2019, 220, S270. [Google Scholar] [CrossRef]
- Wong, T.; Barnes, R.A.; Ross, G.P.; Cheung, N.W.; Flack, J.R. Are the Institute of Medicine Weight Gain Targets Applicable in Women with Gestational Diabetes Mellitus? Diabetologia 2017, 60, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowther, C.A.; Alsweiler, J.M.; Hughes, R.; Brown, J. Tight or Less Tight Glycaemic Targets for Women with Gestational Diabetes Mellitus for Reducing Maternal and Perinatal Morbidity? (TARGET): Study Protocol for a Stepped Wedge Randomised Trial. BMC Pregnancy Childbirth 2018, 18, 425. [Google Scholar] [CrossRef] [Green Version]
- Sam, H.Y.; Skeaff, S.; Skidmore, P. The Validation of a Semi-Quantitative, Multi-Nutrient Food Frequency Questionnaire for Assessing Selected Nutrient Intakes in New Zealand Adults. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2012. [Google Scholar]
- Ministry of Health New Zealand. Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A Background Paper; Ministry of Health New Zealand: Wellington, New Zealand, 2006. [Google Scholar]
- Ministry of Health New Zealand. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline; Ministry of Health New Zealand: Wellington, New Zealand, 2014. [Google Scholar]
- Institute of Medicine and National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13113-1. [Google Scholar]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Huynh, T.; Ghaffari, N.; Bastek, J.; Durnwald, C. Prenatal Care in a Specialized Diabetes in Pregnancy Program Improves Compliance with Postpartum Testing in GDM Women. J. Matern. Neonatal Med. 2017, 30, 1075–1079. [Google Scholar] [CrossRef]
- Martis, R.; Brown, J.; McAra-Couper, J.; Crowther, C.A. Enablers and Barriers for Women with Gestational Diabetes Mellitus to Achieve Optimal Glycaemic Control—A Qualitative Study Using the Theoretical Domains Framework. BMC Pregnancy Childbirth 2018, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Kjøllesdal, M.K.R.; Holmboe-Ottesen, G. Dietary Patterns and Birth Weight—a Review. AIMS Public Health 2014, 1, 211. [Google Scholar] [CrossRef] [PubMed]
- Louvigne, M.; Rouleau, S.; Caldagues, E.; Souto, I.; Montcho, Y.; Bouvagnet, A.M.; Baud, O.; Carel, J.C.; Gascoin, G.; Coutant, R. Association of Maternal Nutrition with Transient Neonatal Hyperinsulinism. PLoS ONE 2018, 13, e0195383. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Plant-Based and Plant-Rich Diet Patterns during Gestation: Beneficial Effects and Possible Shortcomings. Adv. Nutr. 2015, 6, 581. [Google Scholar] [CrossRef] [Green Version]
- Meloncelli, N.; Barnett, A.; de Jersey, S. An Implementation Science Approach for Developing and Implementing a Dietitian-Led Model of Care for Gestational Diabetes: A Pre-Post Study. BMC Pregnancy Childbirth 2020, 20, 661. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.L.; Wall, C.R.; Bloomfield, F.H.; Crowther, C.A. Dietetic Management of Gestational Diabetes in New Zealand: A Cross-Sectional Survey. Nutr. Diet. 2017, 74, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Reader, D.; Splett, P.; Gunderson, E.P. Impact of Gestational Diabetes Mellitus Nutrition Practice Guidelines Implemented by Registered Dietitians on Pregnancy Outcomes. J. Am. Diet. Assoc. 2006, 106, 1426–1433. [Google Scholar] [CrossRef]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Peevy, K.J.; Landaw, S.A.; Gross, S.J. Hyperbilirubinemia in Infants of Diabetic Mothers. Pediatrics 1980, 66, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Khambalia, A.Z.; Algert, C.S.; Bowen, J.R.; Collie, R.J.; Roberts, C.L. Long-Term Outcomes for Large for Gestational Age Infants Born at Term. J. Paediatr. Child Health 2017, 53, 876–881. [Google Scholar] [CrossRef]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef] [Green Version]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, 525–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Griffiths, M.M.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. Excessive Weight Gain Before and During Gestational Diabetes Mellitus Management: What Is the Impact? Diabetes Care 2020, 43, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bevier, W.C.; Jovanovič, L. Weight Gain and Gestational Diabetes Mellitus Is a Sensitive Issue. Diabetes Care 2008, 31, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ouyang, Y.-Q.; Redding, S.R. Maternal Prepregnancy Body Mass Index, Gestational Weight Gain, and Cessation of Breastfeeding: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2019, 14, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.; Grant, C.; Wall, C.; Welch, M.; Marks, E.; Fleming, C.; Teixeira, J.; Bandara, D.; Berry, S.; Morton, S. Breastfeeding Indicators among a Nationally Representative Multi-Ethnic Sample of New Zealand Children. N. Z. Med. J. 2017, 130, 34–44. [Google Scholar] [PubMed]
- Nunnery, D.; Ammerman, A.; Dharod, J. Predictors and Outcomes of Excess Gestational Weight Gain among Low-Income Pregnant Women. Health Care Women Int. 2018, 39, 19–33. [Google Scholar] [CrossRef] [PubMed]


| Maternal Sociodemographics | Adherence to Food-Related Recommendations | Adherence to Non-Food-Related Recommendations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Adherence 2 n (%) | Visited A Dietitian n (%) | Gestational Weight Gain n (%) | |||||||||
| Low (T1) n = 104 | Moderate (T2) n = 105 | High (T3) n = 104 | p-Value | Yes n = 269 | No n = 44 | p Value | Below Recommended n = 110 | Recommended n = 88 | Above Recommended n = 115 | p-Value | |
| Age in years 1 | 33.0 (29.0, 35.0) | 32.0 (29.0, 36.0) | 33.0 (30.0, 36.0) | 0.42 | 33.0 (29.0, 36.0) | 34.0 (30.0, 37.0) | 0.66 | 33.0 (29.0, 36.0) | 34.0 (30.3, 37.0) | 32.0 (30.0, 35.0) | 0.10 |
| ≤20 to 24 years | 5 (4.8) | 3 (2.9) | 5 (4.8) | 12 (4.5) | 1 (2.3) | 2 (1.8) | 4 (4.5) | 7 (6.1) | |||
| 25 to 29 years | 25 (24.0) | 25 (23.8) | 16 (15.4) | 59 (21.9) | 7 (15.9) | 30 (27.3) | 15 (17.0) | 21 (18.3) | |||
| 30 to 34 years | 34 (32.7) | 34 (32.4) | 46 (44.2) | 99 (36.8) | 15 (34.1) | 38 (34.5) | 26 (29.5) | 50 (43.5) | |||
| 35 to 39 years | 31 (29.8) | 35 (33.3) | 25 (24.0) | 74 (27.5) | 17 (38.6) | 28 (25.5) | 33 (37.5) | 30 (26.1) | |||
| ≥40 years | 9 (8.7) | 8 (7.6) | 12 (11.5) | 25 (9.3) | 4 (9.1) | 12 (10.9) | 10 (11.4) | 7 (6.1) | |||
| Ethnicity | 0.97 | 0.16 | 0.25 | ||||||||
| European | 45 (43.3) | 52 (49.5) | 49 (47.1) | 121 (45.0) | 25 (56.8) | 49 (44.5) | 44 (50.0) | 53 (46.1) | |||
| Asian | 37 (35.6) | 31 (29.5) | 30 (28.8) | 86 (32.0) | 12 (27.3) | 35 (31.8) | 32 (36.4) | 31 (27.0) | |||
| Pacific People | 12 (11.5) | 10 (9.5) | 12 (11.5) | 33 (12.3) | 1 (2.3) | 10 (9.1) | 9 (10.2) | 15 (13.0) | |||
| Māori | 9 (8.7) | 11 (10.5) | 11 (10.6) | 26 (9.7) | 5 (11.4) | 14 (12.7) | 2 (2.3) | 15 (13.0) | |||
| Other | 1 (1.0) | 1 (1.0) | 2 (1.9) | 3 (1.1) | 1 (2.3) | 2 (1.8) | 1 (1.1) | 1 (0.9) | |||
| Socioeconomic deprivation index | 0.35 | 0.35 | 0.07 | ||||||||
| 1 to 2 (least deprived) | 16 (15.4) | 26 (24.8) | 26 (25.0) | 54 (20.1) | 14 (31.8) | 17 (15.5) | 26 (38.2) | 25 (21.7) | |||
| 3 to 4 | 21 (20.2) | 15 (14.3) | 10 (9.6) | 40 (14.9) | 6 (13.6) | 16 (14.5) | 8 (17.4) | 22 (19.1) | |||
| 5 to 6 | 12 (11.5) | 14 (13.3) | 17 (16.3) | 40 (14.9) | 3 (6.8) | 14 (12.7) | 8 (18.6) | 21 (18.3) | |||
| 7 to 8 | 23 (22.1) | 18 (17.1) | 17 (16.3) | 49 (18.2) | 9 (20.5) | 22 (20.0) | 18 (31.0) | 18 (15.7) | |||
| 9 to 10 (most deprived) | 32 (30.8) | 32 (30.5) | 34 (32.7) | 86 (32.0) | 12 (27.3) | 41 (37.3) | 28 (28.6) | 29 (25.2) | |||
| Previous GDM | 19 (18.3) | 13 (12.5) | 25 (24.5) | 0.08 | 51 (19.2) | 6 (13.6) | 0.38 | 21 (19.3) | 14 (16.1) | 22 (19.3) | 0.81 |
| Primiparous | 48 (46.2) | 52 (49.5) | 39 (37.5) | 0.20 | 120 (44.6) | 19 (43.2) | 0.86 | 47 (42.7) | 43 (48.9) | 49 (42.6) | 0.61 |
| BMI | 0.63 | 0.25 | 0.10 | ||||||||
| Underweight | 1 (1.0) | 1 (1.0) | 0 (0.00) | 2 (0.7) | 0 (0.0) | 1 (0.9) | 1 (1.1) | 0 (0.0) | |||
| Normal | 8 (7.7) | 11 (10.5) | 12 (11.5) | 30 (11.2) | 1 (2.3) | 17 (15.5) | 9 (10.2) | 5 (4.3) | |||
| Overweight | 29 (27.9) | 37 (35.2) | 37 (35.6) | 86 (32.0) | 17 (38.6) | 32 (29.1) | 33 (37.5) | 38 (33.0) | |||
| Obese | 66 (63.5) | 56 (53.3) | 55 (52.9) | 151 (56.1) | 26 (59.1) | 60 (54.5) | 45 (51.1) | 72 (62.6) | |||
| Family history of diabetes | 53 (53.0) | 54 (54.5) | 62 (61.4) | 0.44 | 139 (54.1) | 30 (69.8) | 0.06 | 57 (54.3) | 52 (61.2) | 60 (54.5) | 0.57 |
| Smoking at trial entry | 11 (10.6) | 9 (8.7) | 6 (5.8) | 0.45 | 24 (9.0) | 2 (4.5) | 0.55 | 8 (7.3) | 6 (6.8) | 12 (10.4) | 0.59 |
| Gestational age at trial entry 1 | 31.4 (30.1, 32.4) | 31.4 (30.3, 32.5) | 31.3 (29.8, 32.3) | 0.78 | 31.3 (29.9, 32.3) | 32.0 (30.8, 33.2) | 0.01 * | 31.2 (30.0, 32.3) | 31.6 (30.0, 32.4) | 31.4 (30.0, 32.6) | 0.86 |
| Health Outcomes | Adherence to Food-Related Recommendations | Adherence to Non-Food-Related Recommendations | ||||||
|---|---|---|---|---|---|---|---|---|
| Dietary Adherence 2 n (%) | Visited a Dietitian n (%) | Gestational Weight Gain n (%) | ||||||
| Low (T1) n = 104 | Moderate (T2) n = 105 | High (T3) n = 104 | Yes n = 269 | No n = 44 | Below Recommended n = 110 | Recommended n = 88 | Above Recommended n = 115 | |
| Maternal health outcomes | ||||||||
| Pre-eclampsia | 4 (3.8) | 5 (4.8) | 6 (5.8) | 11 (4.1) | 4 (9.1) | 8 (7.3) | 3 (3.4) | 4 (3.5) |
| Maternal hypoglycaemia | 1 (1.0) | 4 (3.8) | 1 (1.0) | 5 (1.9) | 1 (2.3) | 4 (3.6) | 0 (0.0) | 2 (1.7) |
| Pharmacological use | ||||||||
| Diet alone | 25 (24.0) | 32 (30.5) | 38 (36.5) | 77 (28.6) | 18 (40.9) | 35 (31.8) | 31 (35.2) | 29 (25.2) |
| Use of oral hypoglycaemics or/and insulin | 79 (76.0) | 73 (69.5) | 66 (63.5) | 192 (71.4) | 26 (59.1) | 75 (68.2) | 57 (64.8) | 86 (74.8) |
| Use of oral hypoglycaemics alone | 32 (40.5) | 32 (43.8) | 30 (45.5) | 80 (41.7) | 14 (53.8) | 33 (44.0) | 28 (49.1) | 33 (38.4) |
| Use of insulin alone | 16 (20.3) | 17 (23.3) | 12 (18.2) | 38 (19.8) | 7 (26.9) | 14 (18.7) | 15 (26.3) | 16 (18.6) |
| Use of oral hypoglycaemics and insulin | 31 (39.2) | 24 (32.9) | 24 (36.4) | 74 (38.5) | 5 (19.3) | 28 (37.3) | 14 (24.6) | 37 (43.0) |
| Labour | 82 (78.8) | 88 (83.8) | 85 (81.7) | 217 (80.7) | 38 (86.4) | 93 (84.5) | 72 (81.8) | 90 (78.3) |
| Spontaneous labour | 26 (31.7) | 21 (23.9) | 36 (42.4) | 70 (32.3) | 13 (34.2) | 29 (31.2) | 26 (36.1) | 28 (31.1) |
| Induced labour | 56 (68.3) | 67 (76.1) | 49 (57.6) | 147 (67.7) | 25 (65.8) | 64 (68.8) | 46 (63.9) | 62 (68.9) |
| Caesarean section | 42 (40.4) | 41 (39.0) | 38 (36.5) | 107 (39.8) | 14 (31.8) | 36 (32.7) | 36 (40.9) | 49 (42.6) |
| Elective caesarean section | 17 (40.5) | 15 (36.6) | 18 (47.4) | 45 (42.1) | 5 (35.7) | 15 (41.7) | 15 (41.7) | 20 (40.8) |
| Emergency caesarean section | 25 (59.5) | 26 (63.4) | 20 (52.6) | 62 (57.9) | 9 (64.3) | 21 (58.3) | 21 (58.3) | 29 (59.2) |
| Postpartum haemorrhage | 28 (27.2) | 25 (24.5) | 18 (18.0) | 65 (24.9) | 6 (13.6) | 17 (15.9) | 25 (29.4) | 29 (25.7) |
| Feeding at hospital discharge | ||||||||
| Breastmilk without formula | 73 (70.2) | 72 (68.6) | 82 (78.8) | 196 (72.9) | 31 (70.5) | 87 (79.1) | 58 (65.9) | 82 (71.3) |
| Both formula and breastmilk | 23 (22.1) | 28 (26.7) | 19 (18.3) | 60 (22.3) | 10 (22.7) | 19 (17.3) | 27 (30.7) | 24 (20.9) |
| Formula without breastmilk | 8 (7.7) | 5 (4.8) | 3 (2.9) | 13 (4.8) | 3 (6.8) | 4 (3.6) | 3 (3.4) | 9 (7.8) |
| Infant health outcomes | ||||||||
| Shoulder dystocia | 1 (1.0) | 1 (1.0) | 2 (1.9) | 4 (1.5) | 0 (0.0) | 0 (0.0) | 2 (2.3) | 2 (1.7) |
| Birthweight in grams, mean (SD) 1 | 3227.6 (493.1) | 3385.5 (475.0) | 3322.6 (494.7) | 3293.2 (479.4) | 3427.7 (543.0) | 3211.6 (469.6) | 3267.6 (479.8) | 3442.40 (493.2) |
| Gestational age at birth <37 weeks | 9 (8.7) | 4 (3.8) | 9 (8.7) | 16 (5.9) | 6 (13.6) | 6 (5.5) | 7 (8.0) | 9 (7.8) |
| Gestational age at birth ≥37 weeks | 95 (91.3) | 101 (96.2) | 95 (91.3) | 253 (94.1) | 38 (86.4) | 104 (94.5) | 81 (92.0) | 106 (92.2) |
| SGA | 9 (8.7) | 3 (2.9) | 10 (9.6) | 19 (7.1) | 3 (6.8) | 11 (10.0) | 8 (9.1) | 3 (2.6) |
| AGA | 85 (81.7) | 91 (86.7) | 82 (78.8) | 227 (84.4) | 31 (70.5) | 90 (81.8) | 74 (84.1) | 94 (81.7) |
| LGA | 10 (9.6) | 11 (10.5) | 12 (11.5) | 23 (8.6) | 10 (22.7) | 9 (8.2) | 6 (6.8) | 18 (15.7) |
| Neonatal hypoglycaemia | 33 (31.7) | 32 (30.5) | 26 (25.0) | 83 (30.9) | 8 (18.2) | 27 (24.5) | 28 (31.8) | 36 (31.3) |
| Hyperbilirubinaemia | 3 (2.9) | 2 (1.9) | 6 (5.8) | 7 (2.6) | 4 (9.1) | 3 (2.7) | 2 (2.3) | 6 (5.2) |
| Any respiratory support after birth | 4 (3.8) | 3 (2.9) | 7 (6.7) | 11 (4.1) | 3 (6.8) | 3 (2.7) | 4 (4.5) | 7 (6.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, S.T.; Harding, J.E.; Wall, C.R.; Crowther, C.A. Adherence to Clinical Practice Guideline Recommendations in Women with Gestational Diabetes and Associations with Maternal and Infant Health—A Cohort Study. Nutrients 2022, 14, 1274. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061274
Mustafa ST, Harding JE, Wall CR, Crowther CA. Adherence to Clinical Practice Guideline Recommendations in Women with Gestational Diabetes and Associations with Maternal and Infant Health—A Cohort Study. Nutrients. 2022; 14(6):1274. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061274
Chicago/Turabian StyleMustafa, Sara T., Jane E. Harding, Clare R. Wall, and Caroline A. Crowther. 2022. "Adherence to Clinical Practice Guideline Recommendations in Women with Gestational Diabetes and Associations with Maternal and Infant Health—A Cohort Study" Nutrients 14, no. 6: 1274. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061274