Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Effect of Probiotics on Viability of HT-29 Colon Cells Using Trypan Blue Assay
2.3. Immunomodulation of TNF-α-Induced HT-29 Cells by BA
2.4. Preparation of Yogurt
2.5. Animal Care and Experimental Design for TNBS-Induced Colitis
2.6. TNBS-Induced Colitis
2.7. Assessment of Disease Activity Index (DAI)
2.8. Bacterial Count in the Fecal Sample
2.9. Tissue Collection and Sample Preparation
2.10. In Vivo Permeability Assay
2.11. Myeloperoxidase (MPO) Activity
2.12. Markers of Inflammation in the Intestinal Tissue
2.13. Transcriptional Expression of Inflammatory Genes
2.14. Western Blot
2.15. Statistical Analysis
3. Results
3.1. Effect of Immunomodulation and Biocompatibility of BA on HT-29 Colon Cell Lines
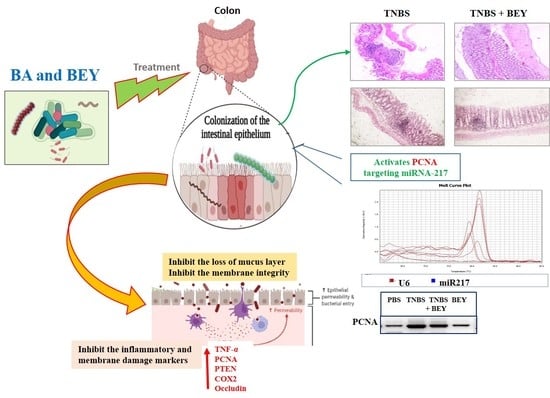
3.2. BEY Alleviated TNBS-Induced Colitis in C57Bl6j Mice
3.3. Effect of BEY on Inflammatory Markers in TNBS-Induced Colitis Mice
3.4. Effect of BEY on Intestinal Barrier Function of TNBS-Induced Colitis Mice
3.5. Immunohistochemical Expression of PCNA in Colon Tissues of TNBS-Induced Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordeiro, B.F.; Lemos, L.; Oliveira, E.R.; Silva, S.H.; Savassi, B.; Figueiroa, A.; Faria, A.M.C.; Ferreira, E.; Esmerino, E.A.; Rocha, R.S.; et al. Prato cheese containing Lactobacillus casei 01 fails to prevent dextran sodium sulphate-induced colitis. Int. Dairy J. 2019, 99, 104551. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns. Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.; Alawadhi, S.; Hasan, F.; Abou Rached, A.; Sanai, F.; Danese, S. Incidence, Prevalence, and Clinical Epidemiology of Inflammatory Bowel Disease in the Arab World: A Systematic Review and Meta-Analysis. Inflamm. Intest. Dis. 2021, 6, 123–131. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, K.; Chen, Q.; Wang, Y. Jirimutu Camel milk modulates the gut microbiota and has anti-inflammatory effects in a mouse model of colitis. J. Dairy Sci. 2022, 105, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- Rabah, H.; Do Carmo, F.L.R.; Carvalho, R.D.d.O.; Cordeiro, B.F.; da Silva, S.H.; Oliveira, E.R.; Lemos, L.; Cara, D.C.; Faria, A.M.C.; Garric, G.; et al. Beneficial propionibacteria within a probiotic emmental cheese: Impact on dextran sodium sulphate-induced colitis in mice. Microorganisms 2020, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.; Guerra, G.; Soares, J.; Santos, K.; Rolim, F.; Assis, P.; Araújo, D.; de Araújo Júnior, R.F.; Garcia, V.B.; de Araújo, A.A.; et al. Lactobacillus rhamnosus EM1107 in goat milk matrix modulates intestinal inflammation involving NF-κB p65 and SOCs-1 in an acid-induced colitis model. J. Funct. Foods 2018, 50, 78–92. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Guo, C.; Geng, M.; Gai, S.; Qi, W.; Li, Z.; Song, Y.; Luo, X.; Zhang, T.; et al. Bacillus subtilis RZ001 improves intestinal integrity and alleviates colitis by inhibiting the Notch signalling pathway and activating ATOH-1. Pathog. Dis. 2020, 78, ftaa016. [Google Scholar] [CrossRef]
- Kang, M.; Choi, H.J.; Yun, B.; Lee, J.; Yoo, J.; Yang, H.J.; Jeong, D.Y.; Kim, Y.; Oh, S. Bacillus amyloliquefaciens SCGB1 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice through Immune Regulation. J. Med. Food 2021, 24, 709–719. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhang, L.; Lu, Z.; Wang, T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Hairul Islam, V.I.; Prakash Babu, N.; Pandikumar, P.; Ignacimuthu, S. Isolation and Characterization of Putative Probiotic Bacterial Strain, Bacillus amyloliquefaciens, from North East Himalayan Soil Based on In Vitro and In Vivo Functional Properties. Probiotics Antimicrob. Proteins 2011, 3, 175–185. [Google Scholar] [CrossRef]
- Hairul Islam, V.I.; Saravanan, S.; Preetam Raj, J.P.; Gabriel Paulraj, M.; Ignacimuthu, S. Myroides pelagicus from the gut of Drosophila melanogaster attenuates inflammation on dextran sodium sulfate-induced colitis. Dig. Dis. Sci. 2014, 59, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Duary, R.K.; Batish, V.K.; Grover, S. Immunomodulatory activity of two potential probiotic strains in LPS-stimulated HT-29 cells. Genes Nutr. 2014, 9, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-dhabi, N.A.; Arasu, M.V.; Vijayaraghavan, P.; Esmail, G.A.; Duraipandiyan, V.; Kim, Y.O.; Kim, H.; Kim, H. Probiotic and Antioxidant Potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 Isolated from Pineapple Puree and Quality Analysis of Pineapple-Flavored Goat Milk Yoghurt during Storage. Microorganisms 2020, 8, 1461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-Induced Model of Chronic Inflammation and Ulceration in the Rat Colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Clayburgh, D.R.; Shen, L.; Turner, J.R. A porous defense: The leaky epithelial barrier in intestinal disease. Lab. Investig. 2004, 84, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-κb activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145–4151. [Google Scholar] [CrossRef]
- Sheikh, A.; Almathen, F.; Ibrahim, H.I.M. Expression of the tyrosinase gene in different dromedary camels of saudi arabia. Pak. J. Zool. 2021, 53, 1939–1945. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.I.M.; El-Fass, K.A.; Akrawi, S.H.; Morsy, M.A. Orientin Alleviates Liver Inflammation via Downregulation of ZEB-2/PTEN Markers—Hepatic Stellate Cells Approach. Appl. Sci. 2022, 12, 2725. [Google Scholar] [CrossRef]
- Fugl, A.; Berhe, T.; Kiran, A.; Hussain, S.; Laursen, M.F.; Bahl, M.I.; Hailu, Y.; Sørensen, K.I.; Guya, M.E.; Ipsen, R.; et al. Characterisation of lactic acid bacteria in spontaneously fermented camel milk and selection of strains for fermentation of camel milk. Int. Dairy J. 2017, 73, 19–24. [Google Scholar] [CrossRef]
- Wen, Y.; He, Q.; Ding, J.; Wang, H.; Hou, Q.; Zheng, Y.; Li, C.; Ma, Y.; Zhang, H.; Kwok, L.Y. Cow, yak, and camel milk diets differentially modulated the systemic immunity and fecal microbiota of rats. Sci. Bull. 2017, 62, 405–414. [Google Scholar] [CrossRef] [Green Version]
- do Carmo, F.L.R.; Rabah, H.; de Oliveira Carvalho, R.D.; Gaucher, F.; Cordeiro, B.F.; da Silva, S.H.; Le Loir, Y.; Azevedo, V.; Jan, G. Extractable bacterial surface proteins in probiotic-host interaction. Front. Microbiol. 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Slaets, H.; De Paepe, K.; Ceulemans, M.; Wetzels, S.; Geboers, K.; Toth, J.; Thys, W.; Dybajlo, R.; Walgraeve, D.; et al. Efficacy and safety of spore-forming probiotics in the treatment of functional dyspepsia: A pilot randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 784–792. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10, 564. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Zhang, X.; Zhu, X. Lactobacillus acidophilus ATCC 4356 Alleviates Renal Ischemia–Reperfusion Injury Through Antioxidant Stress and Anti-inflammatory Responses and Improves Intestinal Microbial Distribution. Front. Nutr. 2021, 8, 149. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lu, Y.; Yue, Y.; Wu, S.; Wang, S.; Yu, M.; Sun, Z. Camel milk regulates T-cell proliferation to alleviate dextran sodium sulphate-induced colitis in mice. Int. J. Food Sci. Technol. 2020, 55, 1648–1660. [Google Scholar] [CrossRef]
- Kangwan, N.; Kongkarnka, S.; Boonkerd, N.; Unban, K.; Shetty, K.; Khanongnuch, C. Protective Effect of Probiotics Isolated from Traditional Fermented Tea Leaves (Miang) from Northern Thailand and Role of Synbiotics in Ameliorating Experimental Ulcerative Colitis in Mice. Nutrients 2022, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Hu, Y.; Yang, Y.; Yuan, J.; Wu, Y.; Li, S.; Lin, J.; He, L.; Hou, S.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107, 2201–2210. [Google Scholar] [CrossRef]
- Kretzmann, N.A.; Fillmann, H.; Mauriz, J.L.; Marroni, C.A.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor Kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm. Bowel Dis. 2008, 14, 1504–1513. [Google Scholar] [CrossRef]
- Darwish, H.A.; Abd Raboh, N.R.; Mahdy, A. Camel’s milk alleviates alcohol-induced liver injury in rats. Food Chem. Toxicol. 2012, 50, 1377–1383. [Google Scholar] [CrossRef]
- Salami, M.; Moosavi-Movahedi, A.A.; Moosavi-Movahedi, F.; Ehsani, M.R.; Yousefi, R.; Farhadi, M.; Niasari-Naslaji, A.; Saboury, A.A.; Chobert, J.M.; Haertlé, T. Biological activity of camel milk casein following enzymatic digestion. J. Dairy Res. 2011, 78, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhu, F.; Xu, B. An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J. Funct. Foods 2018, 47, 334–342. [Google Scholar] [CrossRef]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary Tangeretin Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Inhibiting Inflammatory Response, Restoring Intestinal Barrier Function, and Modulating Gut Microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef]
- Somade, O.T.; Adeyi, O.E.; Ajayi, B.O.; Asunde, O.O.; Iloh, P.D.; Adesanya, A.A.; Babalola, O.I.; Folorunsho, O.T.; Olakunle, D.A.; Lawal, O.F. Syringic and ascorbic acids prevent NDMA-induced pulmonary fibrogenesis, inflammation, apoptosis, and oxidative stress through the regulation of PI3K-Akt/PKB-mTOR-PTEN signaling pathway. Metab. Open 2022, 14, 100179. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhao, S.; Jiang, L.; Lu, L.; Yang, Q.; Yu, Q. Lactobacillus reuteri Stimulates Intestinal Epithelial Proliferation and Induces Differentiation into Goblet Cells in Young Chickens. J. Agric. Food Chem. 2019, 67, 13758–13766. [Google Scholar] [CrossRef] [PubMed]
- Kaeid Sharaf, L.; Shukla, G. Probiotics (Lactobacillus acidophilus and Lactobacillus rhamnosus GG) in Conjunction with Celecoxib (selective COX-2 inhibitor) Modulated DMH-Induced Early Experimental Colon Carcinogenesis. Nutr. Cancer 2018, 70, 946–955. [Google Scholar] [CrossRef]
- Zeng, Q.; He, X.; Puthiyakunnon, S.; Xiao, H.; Gong, Z.; Boddu, S.; Chen, L.; Tian, H.; Huang, S.H.; Cao, H. Probiotic mixture golden Bifido prevents neonatal Escherichia coli K1 translocation via enhancing intestinal defense. Front. Microbiol. 2017, 8, 1798. [Google Scholar] [CrossRef]
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B.; Abraham, C.; Turner, J.R. Targeted Epithelial Tight Junction Dysfunction Causes Immune Activation and Contributes to Development of Experimental Colitis. Gastroenterology 2009, 136, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C.; Wu, J.Q.; Wang, F.; Tang, F.Y.; Sun, J.; Xu, B.; Jiang, M.; Chu, Y.; Chen, D.; Li, X.; et al. QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 2019, 52, e12547. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Su, Y.; Fang, X.; Guo, W. The alleviating effect and mechanism of Bilobalide on ulcerative colitis. Food Funct. 2021, 12, 6226–6239. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Raj Christian, S.D.; Thirugnanasambantham, K.; Islam, M.I.H.; Sudalaimuthu, M.K.; Sundaram, S.; Ashok, G.; Senthilkumar, V.; Muralidaran, S.; Subramanian, S. Identification of Expressed miRNAs in Human Rheumatoid Arthritis Using Computational Approach—Discovery of a New miR-7167 from Human. MicroRNA 2018, 8, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fu, X.; Xie, J.; Pan, H.; Han, W.; Huang, W. miR-26a attenuates colitis and colitis-associated cancer by targeting the multiple intestinal inflammatory pathways. Mol. Ther.-Nucleic Acids 2021, 24, 264–273. [Google Scholar] [CrossRef]





| Primer Name | Forward Primer | Reverse Primer | PCR Product Size in bp |
|---|---|---|---|
| NFκB | CATGAAGAGAAGACACTGACCATGGAAA | TGGATAGAGGCTAAGTGT AGACACG | 245 |
| PTEN | ATGACAGCCATCATCAAAGAGATCGTTAG | GGGTCAGACTTTTGTAATTTGTGAATGCTG | 148 |
| PCNA | TGC TCT GAG GTA CCT GAA CT | TGC TTC CTC ATC TTC AAT CT | 189 |
| COX2 | CAA TTC CCG GAC GTC TAA ACC | CTA GGA CGA TGG GCA TGA AAC | 114 |
| OCCLUDIN | ACG TCC GAC CCA TGC TCT CT | AAG TCA TCC GCA GGG GAG GT | 147 |
| GAPDH | TGGCCTACATGGCCT CCA | TCCCTAGGCCCCTCCTGTTAT | 177 |
| Nutritional Content | BEY—Values in Grams (g) | Commercial Yogurt—Values in (g) |
|---|---|---|
| Total fat | 3.4 ± 0.2 | 3.22 ± 0.13 |
| Trans fat | 0.0 | 0.0 |
| Cholesterol | 0.049 ± 0.01 | 0.051 ± 0.007 |
| Sodium | 0.04 ± 0.01 | 0.035 ± 0.1 |
| Total carbohydrates | 4.5 ± 0.31 | 4.1 ± 0.24 |
| Protein | 3.2 ± 0.28 | 2.92 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.; Sheikh, A.; Ibrahim, H.I.M. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients 2022, 14, 1967. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14091967
Khalifa A, Sheikh A, Ibrahim HIM. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients. 2022; 14(9):1967. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14091967
Chicago/Turabian StyleKhalifa, Ashraf, Abdullah Sheikh, and Hairul Islam Mohamed Ibrahim. 2022. "Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model" Nutrients 14, no. 9: 1967. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14091967