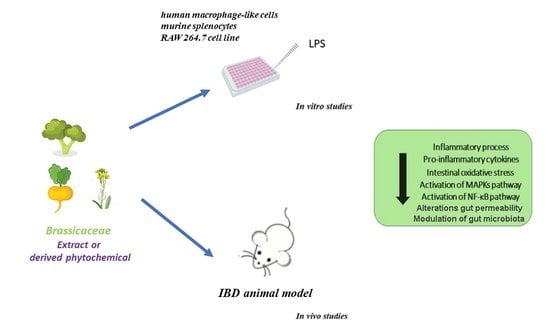
Anti-Inflammatory Potential of Brassicaceae-Derived Phytochemicals: In Vitro and In Vivo Evidence for a Putative Role in the Prevention and Treatment of IBD
Abstract
:1. Introduction
2. Brassicaceae and Inflammation: Evidence of Anti-Inflammatory and Antioxidant Effects In Vitro
2.1. Inflammatory Mediators
2.1.1. Effects of Brassicaceae Extracts
2.1.2. Effects of Brassicaceae-Derived Phytochemicals
2.2. Inflammatory Pathways
2.2.1. Effects of Brassicaceae Extracts
2.2.2. Effects of Brassicaceae-Derived Phytochemicals
2.3. Antioxidant Effects
| Brassicaceae | Type of Extract | Dose | Cell Model | Reported Activity | Reference |
|---|---|---|---|---|---|
| BRASSICACEAE EXTRACTS | |||||
| Iberis amara | Fresh plant extract dissolved in water or in 31% V/V ethanol solution | STW5 128 μg/mL STW6 6 μg/mL | LPS-stimulated monocytes | ↑ IL-10 ↓TNF-α | [55] |
| Radish sprout | Ethanol extract | LPS-stimulated RAW-264.7 | ↓ TNF-α, IL-1β, IL-6, MCP-1 iNOS, COX-2 | [56] | |
| Broccoli (Brassica oleracea var. italia) florets | Methanol extract | 25–50–100 μg/mL | LPS-stimulated RAW-264.7 | ↓ NO, iNOS, TNF-α, IL-1β, IL-6 ↓ IκB-α degradation and NF-κB | [57] |
| Brassica oleracea L. var. capitata | Methanol extract | 25–50–100 μg/mL | LPS-stimulated RAW-264.7 | ↓ NO production | [58] |
| Brassica oleracea L. convar. Botrytis var. cymosa 6-day-sprouts | Methanol/water extract | 75–100 μg/mL | Human PBMC | ↓ TNF-α, IL-6 and IL-1β ↑ IL-10 ↓ NO, iNOS, COX-2, PGE2 | [59] |
| Bok Choy (Brassica campestris var. chinensis) Sprouts | Water extract | 50–100 μg/mL | LPS-stimulated RAW-264.7 | ↓ NO, iNOS, IL-1β, IL-6 and TNF-α ↓ MAPK activation | [65] |
| Brassica napus L. | hydrosols | 1–2.5–5 % | LPS-stimulated RAW-264.7 | ↓ NO, iNOS, COX-2, PGE2, NF-κB | [66] |
| Brassica oleracea L. Convar. acephala Var. sabellica | Methanolic extracts | TNF-α- stimulated HUVEC | ↓ E-selectin, VCAM-1 and ICAM-1 | [67] | |
| BRASSICACAE-DERIVED PHYTOCHEMICALS | |||||
| Brassica | Glucosinates and flavonoids | 25–50 μM | human macrophage-like | ↓ TNF-α, IL-1β, IL-6 | [60] |
| Brassica oleracea. var. capitate | Pigments from juice | 20–100–500 μg/mL | LPS-stimulated murine splenocyte | ↑ IL-10 ↓ IL-6 | [61] |
| Brassica rapa | Arvelexin | 25–50–100 μM | LPS-stimulated RAW-264.7 | ↓ NF-κB activation ↓ TNF-α, IL-6 and IL-1β ↑ IL-10 ↓ NO, iNOS, COX-2, PGE2 | [62] |
| Watercress Nasturtium officinalis | PEITC and MSO | 1–5–10 μM | LPS-stimulated RAW-264.7 | ↓ Metalloproteinase-9 activity and invasiveness | [68] |
| Brassica | SFN | 25–50–100 μM | LPS-stimulated RAW-264.7 | ↓ NO, iNOS, TNF-α, COX-2, PGE2 | [69] |
| Plum cabbage (Kale, Brassica oleracea var. sabellica) | Carotenoids and polyphenols | Digesta (500 mg of dried matrix and 3 g cream) diluted 1:8 with medium | LPS-stimulated Caco-2 LPS-stimulated Caco-2/HT-29-MTX, and THP-1 | ↓ Catalase, glutathione transferase, and SOD | [85] |
3. Brassicaceae and Inflammation: Evidence of Anti-Inflammatory and Antioxidant Effects In Vivo Focusing on IBD
3.1. Effects of Brassicaceae Extracts
3.2. Effects of Brassicaceae-Derived Phytochemicals
| Brassicaceae | Type of Extract | Dose | Animal Model | Reported Activity | Reference |
|---|---|---|---|---|---|
| BRASSICACEAE EXTRACTS | |||||
| Brassica oleracea var. capitata rubra | Ethanol Extract | 5 mg/kg twice daily oral administration | TNBS/ DSS-mice | ↓ inflammatory scores ↓ MPO activity, lipid peroxidation, ↓ IL-1β and TNF-α | [89] |
| Raphanus sativus L. seeds | Water Extract | 100 mg/kg/d oral administration | TNBS/ DSS-model | ↓ MPO, TNF-α, IL-1β, malondialdehyde production monocyte chemotactic protein-1, iNOS and intercellular adhesion molecule-1. ↓ NF-kB activities | [90] |
| Broccoli | BDNs | 0.25 g/mouse/d oral administration | DSS-mice | ↓ TNF-a, IL-17A, and IFN-γ, ↓ CD4+ T ↑ IL-10, activation: AMPK | [91] |
| Wasabia Japonica roots | Ethanol Extract | 20–50–100 mg/kg/d oral administration | DSS-mice | ↓ NF-kB signaling pathway recovery epithelial tight junctions | [92] |
| Camelina sativa | Defatted Seed Meal | 1 g/kg/d oral administration | DNBS-rat | Relieves visceral hypersensitivity Prevents enteric neuron damage Modulates PPAR-α receptors | [94] |
| Eruca sativa | Water Extract | 1 g/kg/d oral administration | DNBS-rat | Visceral anti-nociceptive effect Release of H2S ↑ Kv7 activity | [95] |
| Maca (Lepidium meyenii) | Crude Extract | 100 mg/kg/d Oral gavage | DSS-mice | ↓ Inflammatory scores ↓ MPO, TNF-α, IL-1β, IL-6. ↑ IL-10, intestinal tight junction | [98] |
| Kale (Brassica oleracea) | Diet | 500 mg/kg/d (60%kale + 40% papaya) oral administration | TNBS-rat | ↓ iNOS, TNF-α, IL-1β and MPO activity Modulation of bacterial flora | [99] |
| Broccoli | Supplemented Diet | Diet + 10% | mdr1a−/− mice | Modulation of caecal microbiota composition and metabolism, ↑ colon morphology | [100] |
| Raphanus sativus | Ethanol Extract | 40/70/100 mg/kg/d oral administration | DSS-mice | ↓ COX-2, TNF-α, IL-1β, IL-6, PGE2 and MPO. ↓ IκB phosphorylation | [56] |
| Lepidium virginicum | Ethanol Extract | 3–30–100 mg/kg/d Oral or Intraperitoneal injection | DNBS-rat | ↓ inflammatory score, MPO activity, CXCL-1, ↓ TNF-α, and IL-1β | [101] |
| BRASSICACAE-DERIVED PHYTOCHEMICALS | |||||
| Brassica-derived isothiocyanate sulforaphane | SFN | 25 mg/kg/d oral administration | DSS-mice | ↓ inflammatory scores, ↑ Nrf2 dependent genes | [106] |
| Broccoli | SFN | supplemented diet preparation, either raw or lightly cooked | DSS-mice | ↓ inflammatory scores, DAI ↓ IL-6, V-CAM1 | [107] |
| Wasabia japonica | AITC | 10 mg/kg/d oral administration | DSS-mice | ↑ tight junction proteins, MUC-2 | [108] |
| Wasabia japonica | 6-MITC | 10 mg/kg/d Oral or intraperitoneal injection | DSS-mice | ↓ IL-6, iNOS, NF-kB and colon damage. GSK-3b inhibition | [109] |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hibi, T.; Ogata, H. Novel Pathophysiological Concepts of Inflammatory Bowel Disease. J. Gastroenterol. 2006, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway Paradigms Revealed from the Genetics of Inflammatory Bowel Disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative Colitis. Nat. Rev. Dis. Prim. 2020, 6, 74. [Google Scholar] [CrossRef]
- Qin, X. Why Is Damage Limited to the Mucosa in Ulcerative Colitis but Transmural in Crohn’s Disease? World J. Gastrointest. Pathophysiol. 2013, 4, 63. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Etiopathogenesis of Inflammatory Bowel Diseases. World J. Gastroenterol. 2006, 12, 4807. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Pro-Inflammatory Cytokines in the Pathogenesis of IBD. Gastroenterology 2011, 140, 1756. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes Dev. 2012, 26, 203. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kassam, Z.; Belga, S.; Roifman, I.; Hirota, S.; Jijon, H.; Kaplan, G.G.; Ghosh, S.; Beck, P.L. Inflammatory Bowel Disease Cause-Specific Mortality: A Primer for Clinicians. Inflamm. Bowel Dis. 2014, 20, 2483. [Google Scholar] [CrossRef] [Green Version]
- Kotze, P.G.; Steinwurz, F.; Francisconi, C.; Zaltman, C.; Pinheiro, M.; Salese, L.; Ponce de Leon, D. Review of the Epidemiology and Burden of Ulcerative Colitis in Latin America. Therap. Adv. Gastroenterol. 2020, 13, 1756284820931739. [Google Scholar] [CrossRef]
- May, D.; Pan, S.; Crispin, D.A.; Lai, K.; Bronner, M.P.; Hogan, J.; Hockenbery, D.M.; McIntosh, M.; Brentnall, T.A.; Chen, R. Investigating Neoplastic Progression of Ulcerative Colitis with Label-Free Comparative Proteomics. J. Proteome Res. 2011, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.; Mas, E. Drug Resistance in Inflammatory Bowel Diseases. Curr. Opin. Pharmacol. 2015, 25, 56–61. [Google Scholar] [CrossRef]
- Coskun, M.; Vermeire, S.; Nielsen, O.H. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol. Sci. 2017, 38, 127–142. [Google Scholar] [CrossRef]
- Na, S.Y.; Moon, W. Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polkinghorne, I.; Hamerli, D.; Cowan, P.; Duckworth, J. Plant-Based Immunocontraceptive Control of Wildlife—“potentials, Limitations, and Possums”. Vaccine 2005, 23, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Hamdani, M. Plants Used to Treat Skin Diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 1819. [Google Scholar] [CrossRef]
- Morsy, M.A.; Gupta, S.; Nair, A.B.; Venugopala, K.N.; Greish, K.; El-Daly, M. Protective Effect of Spirulina Platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients 2019, 11, 2309. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Zhai, L.; Peng, J.; Wu, H.; Bian, Z.; Xiao, H. Phytochemicals as Regulators of Th17/Treg Balance in Inflammatory Bowel Diseases. Biomed. Pharmacother. 2021, 141, 111931. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Scoglio, S.; Serio, R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients 2020, 12, 3635. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-Inflammatory and Immunomodulatory Effects of Spirulina Platensis in Comparison to Dunaliella Salina in Acetic Acid-Induced Rat Experimental Colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and Phylogeny of the Brassicaceae (Cruciferae): An Overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Al-shehbaz, I.A. Brassicaceae (Mustard Family). In eLS; John Wiley & Sons, Ltd: Chichester, UK, 2011; pp. 1–8. [Google Scholar] [CrossRef]
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules 2022, 27, 6008. [Google Scholar] [CrossRef]
- Raza, A.; Hafeez, M.B.; Zahra, N.; Shaukat, K.; Umbreen, S.; Tabassum, J.; Charagh, S.; Khan, R.S.A.; Hasanuzzaman, M. The Plant Family Brassicaceae: Introduction, Biology, and Importance. In The Plant Family Brassicaceae; Springer: Singapore, 2020; pp. 1–43. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A Rich Source of Health Improving Phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [Green Version]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Wang, L.I.; Giovannucci, E.L.; Hunter, D.; Neuberg, D.; Su, L.; Christiani, D.C. Dietary Intake of Cruciferous Vegetables, Glutathione S-Transferase (GST) Polymorphisms and Lung Cancer Risk in a Caucasian Population. Cancer Causes Control 2004, 15, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Baek, N.I.; Chung, H.G.; Bang, M.H.; Jeong, T.S.; Tae Lee, K.; Kang, Y.J.; Lee, M.K.; Kim, H.J.; Yeo, J.; et al. Effects of the Ethanol Extract of the Roots of Brassica Rapa on Glucose and Lipid Metabolism in C57BL/KsJ-Db/Db Mice. Clin. Nutr. 2008, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Han, J.-I.; Kim, M.J.; Park, J.S.; Han, J.M.; Baek, N.I.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Jeong, T.S. Ethanolic Extracts of Brassica Campestris Spp. Rapa Roots Prevent High-Fat Diet-Induced Obesity via Beta (3)-Adrenergic Regulation of White Adipocyte Lipolytic Activity. J. Med. Food 2010, 13, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red Cabbage Anthocyanins: Profile, Isolation, Identification, and Antioxidant Activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS Characterization of Flavonoids and Hydroxycinnamic Derivatives in Turnip Tops (Brassica rapa L. Subsp. Sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White Cabbage (Brassica oleracea Var. Capitata f. Alba): Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Schonhof, I.; Krumbein, A.; Brückner, B. Genotypic Effects on Glucosinolates and Sensory Properties of Broccoli and Cauliflower. Nahrung 2004, 48, 25–33. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, Chemical Characterization and Biological Activity Determination of Broccoli Health Promoting Compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Gao, B.; Yu, L.; Wang, T.T.Y.; Sun, J.; Chen, P.; Liu, J.; Yu, L. Chemical Compositions of Cold-Pressed Broccoli, Carrot, and Cucumber Seed Flours and Their in vitro Gut Microbiota Modulatory, Anti-Inflammatory, and Free Radical Scavenging Properties. J. Agric. Food Chem. 2018, 66, 9309–9317. [Google Scholar] [CrossRef]
- Jensen, J.; Styrishave, B.; Gimsing, A.L.; Hansen, H.C.B. The Toxic Effects of Benzyl Glucosinolate and Its Hydrolysis Product, the Biofumigant Benzyl Isothiocyanate, to Folsomia Fimetaria. Environ. Toxicol. Chem. 2010, 29, 359–364. [Google Scholar] [CrossRef]
- Smolinska, U.; Morra, M.J.; Knudsen, G.R.; Brown, P.D. Toxicity of Glucosinolate Degradation Products from Brassica Napus Seed Meal Toward Aphanomyces Euteiches f. Sp. Pisi. Phytopathology 1997, 87, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Holst, B.; Fenwick, G.R. Glucosinolates. Encycl. Food Sci. Nutr. 2003, 2922–2930. [Google Scholar] [CrossRef]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef] [Green Version]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Kandala, P.K.; Wright, S.E.; Srivastava, S.K. Blocking Epidermal Growth Factor Receptor Activation by 3,3′-Diindolylmethane Suppresses Ovarian Tumor Growth In vitro and In vivo. J. Pharmacol. Exp. Ther. 2012, 341, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant Properties and Lipidic Profile as Quality Indexes of Cauliflower (Brassica oleracea L. Var. Botrytis) in Relation to Harvest Time. Food Chem. 2007, 100, 1019–1025. [Google Scholar] [CrossRef]
- Schonhof, I.; Kläring, H.P.; Krumbein, A.; Claußen, W.; Schreiner, M. Effect of Temperature Increase under Low Radiation Conditions on Phytochemicals and Ascorbic Acid in Greenhouse Grown Broccoli. Agric. Ecosyst. Environ. 2007, 119, 103–111. [Google Scholar] [CrossRef]
- Valente Pereira, F.M.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of Temperature and Ontogeny on the Levels of Glucosinolates in Broccoli (Brassica oleracea Var. Italica) Sprouts and Their Effect on the Induction of Mammalian Phase 2 Enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef]
- Scialabba, A.; Salvini, L.; Faqi, A.S.; Bellani, L.M. Tocopherol, Fatty Acid and Phytosterol Content in Seeds of Nine Wild Taxa of Sicilian Brassica (Cruciferae). Plant Biosyst. 2010, 144, 626–633. [Google Scholar] [CrossRef]
- Serhan, C.N. The Resolution of Inflammation: The Devil in the Flask and in the Details. FASEB J. 2011, 25, 1441. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Kelber, O.; Hauschildt, S.; Spanel-Borowski, K.; Nieber, K. Inhibition of Inflammation-Induced Alterations in Rat Small Intestine by the Herbal Preparations STW 5 and STW 6. Phytomedicine 2009, 16, 161–171. [Google Scholar] [CrossRef]
- Kim, G.; Jang, M.; Hwang, I.; Cho, J.; Kim, S. Radish Sprout Alleviates DSS-Induced Colitis via Regulation of NF-KB Signaling Pathway and Modifying Gut Microbiota. Biomed. Pharmacother. 2021, 144, 112365. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lim, S. Bin Antioxidant and Anti-Inflammatory Activities of Broccoli Florets in LPS-Stimulated RAW 264.7 Cells. Prev. Nutr. Food Sci. 2014, 19, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Rokayya, S.; Li, C.J.; Zhao, Y.; Li, Y.; Sun, C.H. Cabbage (Brassica oleracea L. Var. Capitata) Phytochemicals with Antioxidant and Anti-Inflammatory Potential. Asian Pac. J. Cancer Prev. 2013, 14, 6657–6662. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, M.A.; Granica, S.; Kolodziejczyk-Czepas, J.; Magiera, A.; Czerwinska, M.E.; Nowak, P.; Rutkowska, M.; Wasinski, P.; Owczarek, A. Variability of Sinapic Acid Derivatives during Germination and Their Contribution to Antioxidant and Anti-Inflammatory Effects of Broccoli Sprouts on Human Plasma and Human Peripheral Blood Mononuclear Cells. Food Funct. 2020, 11, 7231–7244. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Martínez-Sánchez, M.A.; García-Peñarrubia, P.; Martinez-Esparza, M.; Ramos-Molina, B.; Moreno, D.A. Analysis of the Anti-Inflammatory Potential of Brassica Bioactive Compounds in a Human Macrophage-like Cell Model Derived from HL-60 Cells. Biomed. Pharmacother. 2022, 149, 112804. [Google Scholar] [CrossRef]
- Lin, J.Y.; Li, C.Y.; Hwang, I.F. Characterisation of the Pigment Components in Red Cabbage (Brassica oleracea L. Var.) Juice and Their Anti-Inflammatory Effects on LPS-Stimulated Murine Splenocytes. Food Chem. 2008, 109, 771–781. [Google Scholar] [CrossRef]
- Shin, J.S.; Noh, Y.S.; Lee, Y.S.; Cho, Y.W.; Baek, N.I.; Choi, M.S.; Jeong, T.S.; Kang, E.; Chung, H.G.; Lee, K.T. Arvelexin from Brassica Rapa Suppresses NF-ΚB-Regulated pro-Inflammatory Gene Expression by Inhibiting Activation of IκB Kinase. Br. J. Pharmacol. 2011, 164, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric Oxide and Superoxide in Inflammation and Immune Regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Geum, N.G.; Yeo, J.H.; Yu, J.H.; Choi, M.Y.; Lee, J.W.; Baek, J.K.; Jeong, J.B. In vitro Immunostimulatory Activity of Bok Choy (Brassica Campestris Var. Chinensis) Sprouts in RAW264.7 Macrophage Cells. Korean J. Plant Resour. 2021, 34, 203–215. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, S.R.; Jeong, M.S.; Choi, M.; Park, S.J.; Kim, K.N. Protective Effect of Brassica napus L. Hydrosols against Inflammation Response in RAW 264.7 Cells. Chin. J. Integr. Med. 2021, 27, 273–279. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C. Extracts from Brassica oleracea L. Convar. Acephala Var. Sabellica Inhibit TNF-α Stimulated Neutrophil Adhesion in vitro under Flow Conditions. Food Funct. 2014, 5, 1082–1090. [Google Scholar] [CrossRef]
- Rose, P.; Yen, K.W.; Choon, N.O.; Whiteman, M. Beta-Phenylethyl and 8-Methylsulphinyloctyl Isothiocyanates, Constituents of Watercress, Suppress LPS Induced Production of Nitric Oxide and Prostaglandin E2 in RAW 264.7 Macrophages. Nitric Oxide Biol. Chem. 2005, 12, 237–243. [Google Scholar] [CrossRef]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear Factor Kappa B Is a Molecular Target for Sulforaphane-Mediated Anti-Inflammatory Mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [Green Version]
- Bachiega, P.; Salgado, J.M.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and Antiproliferative Activities in Different Maturation Stages of Broccoli (Brassica oleracea Italica) Biofortified with Selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef]
- Pia, X.L.; Im, H.Y.I.; Yokozawa, T.; Lees, Y.A.; Piao, X.S.; Cho, E.J. Protective effects of broccoli (Brassica oleracea) and its active components against radical-induced oxidative damage. J. Nutr. Sci. Vitaminol. 2005, 51, 142–147. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic Acid Contents of Kale (Brassica oleraceae L. Var. Acephala DC.) Extracts and Their Antioxidant and Antibacterial Activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Yang, L.; Wang, R.; Wang, C. Ultrasonic-Assisted Enzymatic Extraction of Phenolics from Broccoli (Brassica oleracea L. Var. Italica) Inflorescences and Evaluation of Antioxidant Activity in vitro. Food Sci. Technol. Int. 2015, 21, 306–319. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.; Hsieh, P. Bioactive compounds and bioactivities of Brassica oleracea L. var. Italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Bhatt, S.; Singh, B.; Gupta, M. Antioxidant and Prebiotic Potential of Murraya koenigii and Brassica oleracea Var. Botrytis leaves as Food Ingredient. J. Agric. Food Res. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Picchi, V.; Lo Scalzo, R.; Tava, A.; Doria, F.; Argento, S.; Toscano, S.; Treccarichi, S.; Branca, F. Phytochemical Characterization and In vitro Antioxidant Properties of Four Brassica Wild Species from Italy. Molecules 2020, 25, 3495. [Google Scholar] [CrossRef]
- de Oliveira, I.R.N.; Roque, J.V.; Maia, M.P.; Stringheta, P.C.; Teófilo, R.F. New Strategy for Determination of Anthocyanins, Polyphenols and Antioxidant Capacity of Brassica oleracea Liquid Extract Using Infrared Spectroscopies and Multivariate Regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 172–180. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-Pressed Rapeseed (Brassica Napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.C.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoids, Polyphenols and Micronutrient Profiles of Brassica Oleraceae and Plum Varieties and Their Contribution to Measures of Total Antioxidant Capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Chang, J.; Wang, M.; Jian, Y.; Zhang, F.; Zhu, J.; Wang, Q.; Sun, B. Health-Promoting Phytochemicals and Antioxidant Capacity in Different Organs from Six Varieties of Chinese Kale. Sci. Rep. 2019, 9, 20344. [Google Scholar] [CrossRef]
- Chaudhary, A.; Choudhary, S.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Purple Head Broccoli (Brassica oleracea L. Var. Italica Plenck), a Functional Food Crop for Antioxidant and Anticancer Potential. J. Food Sci. Technol. 2018, 55, 1806–1815. [Google Scholar] [CrossRef]
- Usami, A.; Motooka, R.; Takagi, A.; Nakahashi, H.; Okuno, Y.; Miyazawa, M. Chemical Composition, Aroma Evaluation, and Oxygen Radical Absorbance Capacity of Volatile Oil Extracted from Brassica Rapa Cv. “Yukina” Used in Japanese Traditional Food. J. Oleo Sci. 2014, 63, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurilich, A.C.; Jeffery, E.H.; Juvik, J.A.; Wallig, M.A.; Klein, B.P. Antioxidant Capacity of Different Broccoli (Brassica Oleracea) Genotypes Using the Oxygen Radical Absorbance Capacity (ORAC) Assay. J. Agric. Food Chem. 2002, 50, 5053–5057. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.C.; Lee, Y.A.; Hye, H.Y.; Yokozawa, T. Protective Effects of Broccoli (Brassica oleracea) against Oxidative Damage in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2006, 52, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Kaulmann, A.; Planchon, S.; Renaut, J.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Proteomic Response of Inflammatory Stimulated Intestinal Epithelial Cells to: In vitro Digested Plums and Cabbages Rich in Carotenoids and Polyphenols. Food Funct. 2016, 7, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.V.; Kumar, P. Animal Models of Inflammatory Bowel Disease: A Review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef]
- Baydi, Z.; Limami, Y.; Khalki, L.; Zaid, N.; Naya, A.; Mtairag, E.M.; Oudghiri, M.; Zaid, Y. An Update of Research Animal Models of Inflammatory Bowel Disease. Sci. World J. 2021, 2021, 7479540. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.R.; Panchal, S.K.; Brown, L. Nutraceuticals in Rodent Models as Potential Treatments for Human Inflammatory Bowel Disease. Pharmacol. Res. 2018, 132, 99–107. [Google Scholar] [CrossRef]
- Zielińska, M.; Lewandowska, U.; Podsedek, A.; Cygankiewicz, A.I.; Jacenik, D.; Sałaga, M.; Kordek, R.; Krajewska, W.M.; Fichna, J. Orally Available Extract from Brassica oleracea Var. Capitata Rubra Attenuates Experimental Colitis in Mouse Models of Inflammatory Bowel Diseases. J. Funct. Foods 2015, 17, 587–599. [Google Scholar] [CrossRef]
- Choi, K.C.; Cho, S.W.; Kook, S.H.; Chun, S.R.; Bhattarai, G.; Poudel, S.B.; Kim, M.K.; Lee, K.Y.; Lee, J.C. Intestinal Anti-Inflammatory Activity of the Seeds of Raphanus sativus L. in Experimental Ulcerative Colitis Models. J. Ethnopharmacol. 2016, 179, 55–65. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Choi, S.; Jang, J.E.; Ramalingam, P.; Ko, Y.T.; Kim, S.Y.; Oh, S.H. Wasabia Japonica Is a Potential Functional Food to Prevent Colitis via Inhibiting the NF-ΚB Signaling Pathway. Food Funct. 2017, 8, 2865–2874. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Bhattacharya, A.; Khare, S.K.; Kaushik, G. Camelina Sativa: An Emerging Biofuel Crop. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 2889–2925. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina Sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L.; et al. Beneficial Effects of Eruca Sativa Defatted Seed Meal on Visceral Pain and Intestinal Damage Resulting from Colitis in Rats. Foods 2022, 11, 580. [Google Scholar] [CrossRef]
- Zha, S.; Zhao, Q.; Chen, J.; Wang, L.; Zhang, G.; Zhang, H.; Zhao, B. Extraction, Purification and Antioxidant Activities of the Polysaccharides from Maca (Lepidium meyenii). Carbohydr. Polym. 2014, 111, 584–587. [Google Scholar] [CrossRef]
- Chang, Y.; Lu, W.; Chu, Y.; Yan, J.; Wang, S.; Xu, H.; Ma, H.; Ma, J. Extraction of Polysaccharides from Maca: Characterization and Immunoregulatory Effects on CD4+ T Cells. Int. J. Biol. Macromol. 2020, 154, 477–485. [Google Scholar] [CrossRef]
- Zha, R.; Ge, E.; Guo, L.; Gao, Q.; Lin, Q.; Zhou, W.; Jin, X.; Xie, W.; Yin, H.; Liu, T. A Newly Identified Polyunsaturated Macamide Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Fitoterapia 2021, 152, 104916. [Google Scholar] [CrossRef]
- Lima de Albuquerque, C.; Comalada, M.; Camuesco, D.; Rodríguez-Cabezas, M.E.; Luiz-Ferreira, A.; Nieto, A.; Monteiro de Souza Brito, A.R.; Zarzuelo, A.; Gálvez, J. Effect of Kale and Papaya Supplementation in Colitis Induced by Trinitrobenzenesulfonic Acid in the Rat. e-SPEN 2010, 5, e111–e116. [Google Scholar] [CrossRef] [Green Version]
- Paturi, G.; Mandimika, T.; Butts, C.A.; Zhu, S.; Roy, N.C.; McNabb, W.C.; Ansell, J. Influence of Dietary Blueberry and Broccoli on Cecal Microbiota Activity and Colon Morphology in Mdr1a -/- Mice, a Model of Inflammatory Bowel Diseases. Nutrition 2012, 28, 324–330. [Google Scholar] [CrossRef]
- Cruz-Muñoz, J.R.; Barrios-García, T.; Valdez-Morales, E.E.; Durán-Vazquez, M.F.; Méndez-Rodríguez, K.B.; Barajas-Espinosa, A.; Ochoa-Cortes, F.; Martínez-Saldaña, M.C.; Gómez-Aguirre, Y.A.; Alba, R.G. Ethanolic Extract from Lepidium virginicum L. Ameliorates DNBS-Induced Colitis in Rats. J. Ethnopharmacol. 2022, 289, 115056. [Google Scholar] [CrossRef]
- Al-Bakheit, A.; Abu-Qatouseh, L. Sulforaphane from Broccoli Attenuates Inflammatory Hepcidin by Reducing IL-6 Secretion in Human HepG2 Cells. J. Funct. Foods 2020, 75, 104210. [Google Scholar] [CrossRef]
- Miekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R. Sulphur-Containing Compounds. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 25–46. [Google Scholar] [CrossRef]
- Gasper, A.V.; Al-Janobi, A.; Smith, J.A.; Bacon, J.R.; Fortun, P.; Atherton, C.; Taylor, M.A.; Hawkey, C.J.; Barrett, D.A.; Mithen, R.F. Glutathione S-Transferase M1 Polymorphism and Metabolism of Sulforaphane from Standard and High-Glucosinolate Broccoli. Am. J. Clin. Nutr. 2005, 82, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.E.; Will, O.; Sturm, C.; Lipinski, S.; Rosenstiel, P.; Rimbach, G. DSS-Induced Acute Colitis in C57BL/6 Mice Is Mitigated by Sulforaphane Pre-Treatment. J. Nutr. Biochem. 2013, 24, 2085–2091. [Google Scholar] [CrossRef]
- Wang, Y.; Jeffery, E.H.; Miller, M.J.; Wallig, M.A.; Wu, Y. Lightly Cooked Broccoli Is as Effective as Raw Broccoli in Mitigating Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2018, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.H.; Oh, S.H. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef] [Green Version]
- Lohning, A.; Kidachi, Y.; Kamiie, K.; Sasaki, K.; Ryoyama, K.; Yamaguchi, H. 6- (Methylsulfinyl) Hexyl Isothiocyanate (6-MITC) from Wasabia Japonica Alleviates Inflammatory Bowel Disease (IBD) by Potential Inhibition of Glycogen Synthase Kinase 3 Beta (GSK-3β). Eur. J. Med. Chem. 2021, 216, 113250. [Google Scholar] [CrossRef]
- Lin, S.C.; Cheifetz, A.S. The Use of Complementary and Alternative Medicine in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. (N. Y). 2018, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Lampe, J.W. Brassica Vegetables and Prostate Cancer Risk: A Review of the Epidemiological Evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Campos, H. GSTT1 Genotype Modifies the Association between Cruciferous Vegetable Intake and the Risk of Myocardial Infarction. Am. J. Clin. Nutr. 2007, 86, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.C.; Koh, W.P.; Yuan, J.M.; Qin, L.Q.; Van Dam, R.M. Green Leafy and Cruciferous Vegetable Consumption and Risk of Type 2 Diabetes: Results from the Singapore Chinese Health Study and Meta-Analysis. Br. J. Nutr. 2018, 119, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.; Han, D.Y.; Triggs, C.M.; Fraser, A.G.; Ferguson, L.R. Brassicaceae: Nutrient Analysis and Investigation of Tolerability in People with Crohn’s Disease in a New Zealand Study. Funct. Foods Heal. Dis. 2012, 2, 460–486. [Google Scholar] [CrossRef]
- Laing, B.; Han, D.Y.; Ferguson, L.R. Candidate Genes Involved in Beneficial or Adverse Responses to Commonly Eaten Brassica Vegetables in a New Zealand Crohn’s Disease Cohort. Nutrients 2013, 5, 5046–5064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicio, A.; Serio, R.; Zizzo, M.G. Anti-Inflammatory Potential of Brassicaceae-Derived Phytochemicals: In Vitro and In Vivo Evidence for a Putative Role in the Prevention and Treatment of IBD. Nutrients 2023, 15, 31. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15010031
Cicio A, Serio R, Zizzo MG. Anti-Inflammatory Potential of Brassicaceae-Derived Phytochemicals: In Vitro and In Vivo Evidence for a Putative Role in the Prevention and Treatment of IBD. Nutrients. 2023; 15(1):31. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15010031
Chicago/Turabian StyleCicio, Adele, Rosa Serio, and Maria Grazia Zizzo. 2023. "Anti-Inflammatory Potential of Brassicaceae-Derived Phytochemicals: In Vitro and In Vivo Evidence for a Putative Role in the Prevention and Treatment of IBD" Nutrients 15, no. 1: 31. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15010031