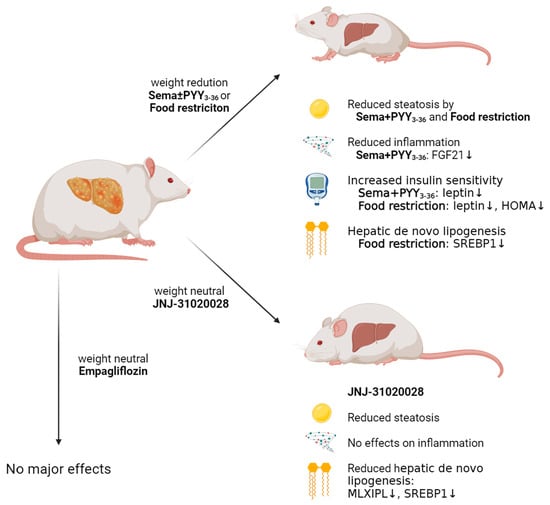
Effects of NPY-2 Receptor Antagonists, Semaglutide, PYY3-36, and Empagliflozin on Early MASLD in Diet-Induced Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Drugs and Treatment
2.2. Dissection and Sample Collection
2.3. Liver Histology
2.4. Enzyme-Linked Immunosorbent Assay and Serum Measurements
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Body Weight Change
3.2. Liver Weight and Histological Assessment: All Agonistic and Antagonistic Incretin-Based Treatments Improved Liver Steatosis Compared to a High-Fat Diet
3.3. Assessment of Inflammation Markers Using Histological Assessment and QrtPCR
3.4. Metabolic Measurements: Insulin Sensitivity Was Ameliorated and Leptin Was Decreased in Semaglutide and PYY3-36-Treated Animals, while Transaminases Did Not Show Any Differences
3.5. Lipogenesis and Lipolysis in the Liver and the Visceral Adipose Tissue
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| BWM | body-weight-matched |
| CH | cholesterol |
| DIO | diet-induced obese |
| DNL | de novo lipogenesis |
| GIP | gastric inhibitory polypeptide |
| GLP-1 | glucagon-like Peptide-1 |
| GLUT4 | glucose transporter type 4 |
| HFD | high-fat diet |
| HOMA | homeostasis model assessment |
| IR | insulin resistance |
| MASLD | metabolic-dysfunction-associated steatotic liver disease |
| MASH | metabolic-associated steatohepatitis |
| NEFA | non-esterified fatty acids |
| NPY | neuropeptide Y |
| NRQs | normalized relative quantities |
| PYY3-36 | peptide YY 3-36 |
| QrtPCR | quantitatively real-time polymerase chain reaction |
| RNA | ribonucleic acid |
| SGLT2 | sodium glucose transporter 2 |
| T2DM | type 2 diabetes mellitus |
| TG | triglyceride |
| Y1R | NPY-1 receptor |
| Y2R | NPY-2 receptor |
References
- Golabi, P.; Paik, J.M.; AlQahtani, S.; Younossi, Y.; Tuncer, G.; Younossi, Z.M. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009–2019. J. Hepatol. 2021, 75, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Li, A.A.; Gadiparthi, C.; Khan, M.A.; Cholankeril, G.; Glenn, J.S.; Ahmed, A. Changing Trends in Etiology-Based Annual Mortality from Chronic Liver Disease, from 2007 through 2016. Gastroenterology 2018, 155, 1154–1163.e1153. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017, 42, 92–108. [Google Scholar] [CrossRef]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Fruhbeck, G.; Catalan, V.; Rodriguez, A.; Ramirez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gomez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 83–92. [Google Scholar] [CrossRef]
- Jitrapakdee, S. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int. J. Biochem. Cell Biol. 2012, 44, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Beaton, M.D. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can. J. Gastroenterol. 2012, 26, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, T.K.; Mhaskar, R.; Schwitalla, T.; Muradova, E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves nonalcoholic fatty liver disease: A contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Reis-Barbosa, P.H.; Marcondes-de-Castro, I.A.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101922. [Google Scholar] [CrossRef]
- Taheri, H.; Malek, M.; Ismail-Beigi, F.; Zamani, F.; Sohrabi, M.; Reza Babaei, M.; Khamseh, M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020, 37, 4697–4708. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(−/−) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- Zhang, W.; Cline, M.A.; Gilbert, E.R. Hypothalamus-adipose tissue crosstalk: Neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 2014, 11, 27. [Google Scholar] [CrossRef]
- Mullins, D.; Kirby, D.; Hwa, J.; Guzzi, M.; Rivier, J.; Parker, E. Identification of potent and selective neuropeptide Y Y(1) receptor agonists with orexigenic activity in vivo. Mol. Pharmacol. 2001, 60, 534–540. [Google Scholar] [PubMed]
- Ishihara, A.; Tanaka, T.; Kanatani, A.; Fukami, T.; Ihara, M.; Fukuroda, T. A potent neuropeptide Y antagonist, 1229U91, suppressed spontaneous food intake in Zucker fatty rats. Am J Physiol 1998, 274, R1500–R1504. [Google Scholar] [CrossRef]
- Kanatani, A.; Ishihara, A.; Asahi, S.; Tanaka, T.; Ozaki, S.; Ihara, M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: Blockade of neuropeptide Y-induced and physiological food intake. Endocrinology 1996, 137, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, A.; Joshi, R.; Su, C.; Friend, L.A.; James, J.H. Neuropeptide Y (NPY) Y2 receptor-selective agonist inhibits food intake and promotes fat metabolism in mice: Combined anorectic effects of Y2 and Y4 receptor-selective agonists. Peptides 2007, 28, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, A.; Schwarzer, C.; Couzens, M.; Fetissov, S.; Furtinger, S.; Jenkins, A.; Cox, H.M.; Sperk, G.; Hokfelt, T.; Herzog, H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8938–8943. [Google Scholar] [CrossRef] [PubMed]
- Oertel, M.; Ziegler, C.G.; Kohlhaas, M.; Nickel, A.; Kloock, S.; Maack, C.; Sequeira, V.; Fassnacht, M.; Dischinger, U. GLP-1 and PYY for the treatment of obesity: Use of agonists and antagonists in diet-induced rats, a pilot study. Endocr. Connect. 2024, 13, e230398. [Google Scholar] [CrossRef]
- Park, S.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines 2021, 9, 1739. [Google Scholar] [CrossRef]
- Shi, Y.C.; Lin, S.; Wong, I.P.; Baldock, P.A.; Aljanova, A.; Enriquez, R.F.; Castillo, L.; Mitchell, N.F.; Ye, J.M.; Zhang, L.; et al. NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS ONE 2010, 5, e11361. [Google Scholar] [CrossRef]
- Dischinger, U.; Corteville, C.; Otto, C.; Fassnacht, M.; Seyfried, F.; Hankir, M.K. GLP-1 and PYY(3-36) reduce high-fat food preference additively after Roux-en-Y gastric bypass in diet-induced obese rats. Surg. Obes. Relat. Dis. 2019, 15, 1483–1492. [Google Scholar] [CrossRef]
- Jones, E.S.; Nunn, N.; Chambers, A.P.; Ostergaard, S.; Wulff, B.S.; Luckman, S.M. Modified Peptide YY Molecule Attenuates the Activity of NPY/AgRP Neurons and Reduces Food Intake in Male Mice. Endocrinology 2019, 160, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, S.M.; D’Aquino, K.; Zhang, Y.M.; Bader, L.; Edwards, W.; Zheng, S.; Eckardt, A.; Lacombe, A.; Pick, R.; Moreno, V.; et al. A Long-Acting PYY(3-36) Analog Mediates Robust Anorectic Efficacy with Minimal Emesis in Nonhuman Primates. Cell Metab. 2019, 29, 837–843.e835. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.B.; Gregersen, N.T.; Pedersen, S.D.; Arentoft, J.L.; Ritz, C.; Schwartz, T.W.; Holst, J.J.; Astrup, A.; Sjodin, A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1248–E1256. [Google Scholar] [CrossRef] [PubMed]
- Metzner, V.; Herzog, G.; Heckel, T.; Bischler, T.; Hasinger, J.; Otto, C.; Fassnacht, M.; Geier, A.; Seyfried, F.; Dischinger, U. Liraglutide + PYY(3-36) Combination Therapy Mimics Effects of Roux-en-Y Bypass on Early NAFLD Whilst Lacking-Behind in Metabolic Improvements. J. Clin. Med. 2022, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Bengtsson, M.; Stahlberg, A.; Rorsman, P.; Kubista, M. Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005, 15, 1388–1392. [Google Scholar] [CrossRef]
- Ailanen, L.; Vahatalo, L.H.; Salomaki-Myftari, H.; Makela, S.; Orpana, W.; Ruohonen, S.T.; Savontaus, E. Peripherally Administered Y(2)-Receptor Antagonist BIIE0246 Prevents Diet-Induced Obesity in Mice with Excess Neuropeptide Y, but Enhances Obesity in Control Mice. Front. Pharmacol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Mercer, R.E.; Chee, M.J.; Colmers, W.F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocr. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Ruohonen, S.T.; Vahatalo, L.H.; Savontaus, E. Diet-induced obesity in mice overexpressing neuropeptide y in noradrenergic neurons. Int. J. Pept. 2012, 2012, 452524. [Google Scholar] [CrossRef]
- Zhang, L.; Riepler, S.J.; Turner, N.; Enriquez, R.F.; Lee, I.C.; Baldock, P.A.; Herzog, H.; Sainsbury, A. Y2 and Y4 receptor signaling synergistically act on energy expenditure and physical activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1618–R1628. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Izaola, O.; de la Fuente, B.; Primo, D.; Aller, R. Association of Neuropeptide Y Gene rs16147 Polymorphism with Cardiovascular Risk Factors, Adipokines, and Metabolic Syndrome in Patients with Obesity. Lifestyle Genom. 2016, 9, 213–221. [Google Scholar] [CrossRef]
- Aller, R.; Lopez-Gomez, J.J.; Izaola, O.; Primo, D.; de Luis, D. Role of neuropeptide Y gene variant (rs161477) in liver histology in obese patients with non-alcoholic fatty liver disease. Endocrinol. Diabetes Nutr. 2019, 66, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, Y.; Yang, K.; Shen, M.; Wang, Y. NPY stimulates cholesterol synthesis acutely by activating the SREBP2-HMGCR pathway through the Y1 and Y5 receptors in murine hepatocytes. Life Sci. 2020, 262, 118478. [Google Scholar] [CrossRef]
- Ortiz, C.; Klein, S.; Reul, W.H.; Magdaleno, F.; Groschl, S.; Dietrich, P.; Schierwagen, R.; Uschner, F.E.; Torres, S.; Hieber, C.; et al. Neprilysin-dependent neuropeptide Y cleavage in the liver promotes fibrosis by blocking NPY-receptor 1. Cell Rep. 2023, 42, 112059. [Google Scholar] [CrossRef] [PubMed]
- Redrobe, J.P.; Dumont, Y.; Herzog, H.; Quirion, R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: Evidence from Y2 receptor knockout mice. Behav. Brain Res. 2003, 141, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, R.; Thorsell, A.; Heilig, M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: Evidence for sensitization in rats with a history of dependence. Neurosci. Lett. 2005, 375, 129–133. [Google Scholar] [CrossRef]
- Noe, F.; Vaghi, V.; Balducci, C.; Fitzsimons, H.; Bland, R.; Zardoni, D.; Sperk, G.; Carli, M.; During, M.J.; Vezzani, A. Anticonvulsant effects and behavioural outcomes of rAAV serotype 1 vector-mediated neuropeptide Y overexpression in rat hippocampus. Gene Ther. 2010, 17, 643–652. [Google Scholar] [CrossRef]
- Brumovsky, P.; Shi, T.S.; Landry, M.; Villar, M.J.; Hokfelt, T. Neuropeptide tyrosine and pain. Trends Pharmacol. Sci. 2007, 28, 93–102. [Google Scholar] [CrossRef]
- Brothers, S.P.; Wahlestedt, C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol. Med. 2010, 2, 429–439. [Google Scholar] [CrossRef]
- Yu, H.H.; Wang, H.C.; Hsieh, M.C.; Lee, M.C.; Su, B.C.; Shan, Y.S. Exendin-4 Attenuates Hepatic Steatosis by Promoting the Autophagy-Lysosomal Pathway. Biomed. Res. Int. 2022, 2022, 4246086. [Google Scholar] [CrossRef]
- Lin, C.; Fang, J.; Xiang, Q.; Zhou, R.; Yang, L. Exendin-4 promotes autophagy to relieve lipid deposition in a NAFLD cell model by activating AKT/mTOR signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mells, J.E.; Fu, P.P.; Saxena, N.K.; Anania, F.A. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS ONE 2011, 6, e25269. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.; Li, H.; Long, X.; Rye, K.A.; Ong, K.L. Fibroblast growth factor 21 in non-alcoholic fatty liver disease. Metabolism 2019, 101, 153994. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K.; Fernandez Lando, L.; Bray, R.; Brouwers, B.; Rodriguez, A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Laker, R.C.; Mather, K.; Nawrocki, A.; Oldham, S.; Boland, B.B.; Lewis, H.; Conway, J.; Naylor, J.; Guionaud, S.; et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat. Metab. 2020, 2, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Uta, S.; Otsubo, M.; Deguchi, Y.; Tagawa, R.; Mizunoe, Y.; Nakagawa, Y.; Shimano, H.; Higami, Y. Srebp-1c/Fgf21/Pgc-1alpha Axis Regulated by Leptin Signaling in Adipocytes-Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients 2020, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.; Cohen, P.; Socci, N.D.; Friedman, J.M. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000, 14, 963–980. [Google Scholar] [CrossRef]
- Luo, J.; Sun, P.; Wang, Y.; Chen, Y.; Niu, Y.; Ding, Y.; Xu, N.; Zhang, Y.; Xie, W. Dapagliflozin attenuates steatosis in livers of high-fat diet-induced mice and oleic acid-treated L02 cells via regulating AMPK/mTOR pathway. Eur. J. Pharmacol. 2021, 907, 174304. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Loffredo, G.; Vetrano, E.; Medicamento, G.; Acierno, C.; Rinaldi, L.; Marrone, A.; Salvatore, T.; Monda, M.; et al. Effects of a Combination of Empagliflozin Plus Metformin vs. Metformin Monotherapy on NAFLD Progression in Type 2 Diabetes: The IMAGIN Pilot Study. Biomedicines 2023, 11, 322. [Google Scholar] [CrossRef]
- Lauritsen, K.M.; Voigt, J.H.; Pedersen, S.B.; Hansen, T.K.; Moller, N.; Jessen, N.; Gormsen, L.C.; Sondergaard, E. Effects of SGLT2 inhibition on lipid transport in adipose tissue in type 2 diabetes. Endocr. Connect. 2022, 11, e210558. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloock, S.; Haerting, N.; Herzog, G.; Oertel, M.; Geiger, N.; Geier, A.; Sequeira, V.; Nickel, A.; Kohlhaas, M.; Fassnacht, M.; et al. Effects of NPY-2 Receptor Antagonists, Semaglutide, PYY3-36, and Empagliflozin on Early MASLD in Diet-Induced Obese Rats. Nutrients 2024, 16, 904. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060904
Kloock S, Haerting N, Herzog G, Oertel M, Geiger N, Geier A, Sequeira V, Nickel A, Kohlhaas M, Fassnacht M, et al. Effects of NPY-2 Receptor Antagonists, Semaglutide, PYY3-36, and Empagliflozin on Early MASLD in Diet-Induced Obese Rats. Nutrients. 2024; 16(6):904. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060904
Chicago/Turabian StyleKloock, Simon, Niklas Haerting, Gloria Herzog, Marie Oertel, Niklas Geiger, Andreas Geier, Vasco Sequeira, Alexander Nickel, Michael Kohlhaas, Martin Fassnacht, and et al. 2024. "Effects of NPY-2 Receptor Antagonists, Semaglutide, PYY3-36, and Empagliflozin on Early MASLD in Diet-Induced Obese Rats" Nutrients 16, no. 6: 904. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060904