Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants—A Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
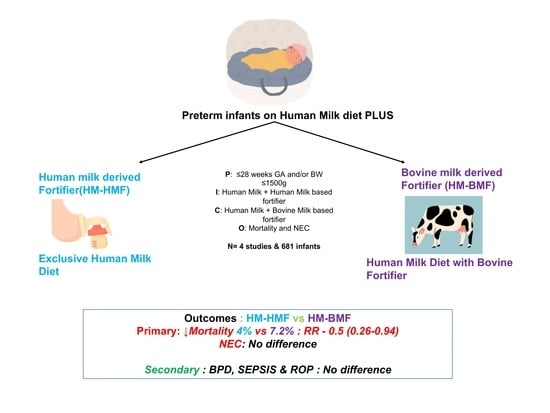
- Infants born after ≤28 weeks of gestation and/or with birthweight ≤ 1500 g were considered for inclusion. Studies comparing the effects of an exclusive human milk diet with human milk-derived fortifiers to the exposure to human milk fortified with bovine milk-derived fortifiers were included.
- Outcomes included NEC, sepsis, or LOS, BPD, ROP, death, feeding intolerance, and growth velocity.
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Definitions and Measurements
- NEC
- BPD
- Sepsis/LOS
- ROP
- Feeding intolerance
- Growth velocity
2.6. Assessment of Methodological Quality
2.7. Summary Measures
2.7.1. The study by Jensen et al. [14] [NORDIC]
2.7.2. OptiMoM Trial [15]
2.7.3. Subgroup Analysis of NCT00506584 [Sullivan trial] from Lucas et al. [16]
2.7.4. Subgroup Analysis of NCT00506584 [Assad trial] from Lucas et al. [17]
2.8. Data Synthesis and Statistical Analysis
3. Results
3.1. Primary Outcomes
3.1.1. Death
3.1.2. NEC
3.2. Secondary Outcomes
3.2.1. BPD
3.2.2. Sepsis
3.2.3. ROP
3.2.4. Feed Intolerance and Growth Velocity
3.2.5. Risk of Bias Assessment and GRADE
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kramer, B.W.; Niklas, V.; Abman, S. Bronchopulmonary Dysplasia and Impaired Neurodevelopment-What May Be the Missing Link? Am. J. Perinatol. 2022, 39, S14–S17. [Google Scholar] [CrossRef]
- Kelly, C.E.; Shaul, M.; Thompson, D.K.; Mainzer, R.M.; Yang, J.Y.M.; Dhollander, T.; Cheong, J.L.Y.; Inder, T.E.; Doyle, L.W.; Anderson, P.J. Long-lasting effects of very preterm birth on brain structure in adulthood: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 147, 105082. [Google Scholar] [CrossRef]
- Diggikar, S.; Gurumoorthy, P.; Trif, P.; Mudura, D.; Nagesh, N.K.; Galis, R.; Vinekar, A.; Kramer, B.W. Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1055813. [Google Scholar] [CrossRef]
- Lu, J.; Martin, C.R.; Claud, E.C. Neurodevelopmental outcome of infants who develop necrotizing enterocolitis: The gut-brain axis. Semin. Perinatol. 2023, 47, 151694. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.; Georgieff, M.; Ramel, S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Martina, F.; Cristina, T.; Massimiliano, C.; Serena Di, M.; Paolo, G. Neurodevelopmental Outcome in Very Low Birth Weight Preterm Newborns: The Role of Neonatal Sepsis. J. Infect. Dis. Epidemiol. 2019, 5, 088. [Google Scholar] [CrossRef]
- Oluwole, I.; Tan, J.B.C.; DeSouza, S.; Hutchinson, M.; Leigh, R.M.; Cha, M.; Rodriguez, A.; Hou, G.; Rao, S.S.; Narang, A.; et al. The association between bronchopulmonary dysplasia grade and risks of adverse neurodevelopmental outcomes among preterm infants born at less than 30 weeks of gestation. J. Matern.-Fetal Neonatal Med. 2023, 36, 2167074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.E.; Vohr, B.R. Neurodevelopmental Outcomes of Preterm Infants Fed Human Milk: A Systematic Review. Clin. Perinatol. 2017, 44, 69–83. [Google Scholar] [CrossRef]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2022, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Cole, T.J. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Domellöf, M.; Ahlsson, F.; Elfvin, A.; Navér, L.; Abrahamsson, T. Effect of Human Milk-Based Fortification in Extremely Preterm Infants Fed Exclusively with Breast Milk: A Randomised Controlled Trial. Available at SSRN. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4529245 (accessed on 31 August 2023).
- O’Connor, D.L.; Kiss, A.; Tomlinson, C.; Bando, N.; Bayliss, A.; Campbell, D.M.; Daneman, A.; Francis, J.; Kotsopoulos, K.; Shah, P.S.; et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: A randomized clinical trial. Am. J. Clin. Nutr. 2018, 108, 108–116. [Google Scholar] [CrossRef]
- Lucas, A.; Boscardin, J.; Abrams, S.A. Preterm Infants Fed Cow’s Milk-Derived Fortifier Had Adverse Outcomes Despite a Base Diet of Only Mother’s Own Milk. Breastfeed Med. 2020, 15, 297–303. [Google Scholar] [CrossRef]
- Lucas, A.; Assad, M.; Sherman, J.; Boscardin, J.; Abrams, S. Safety of Cow’s Milk-Derived Fortifiers Used with an All-Human Milk Base Diet in Very Low Birthweight Preterm Infants. Neonatol. Today 2020, 15, 3–13. [Google Scholar] [CrossRef]
- Assad, M.; Elliott, M.J.; Abraham, J.H. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J. Perinatol. 2016, 36, 216–220. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: https://web.archive.org/web/20210716121605id_/http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 30 October 2023).
- Georg Bach, J.; Fredrik, A.; Magnus, D.; Anders, E.; Lars, N.; Thomas, A. Nordic study on human milk fortification in extremely preterm infants: A randomised controlled trial—The N-forte trial. BMJ Open 2021, 11, e053400. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An Exclusively Human Milk-Based Diet Is Associated with a Lower Rate of Necrotizing Enterocolitis than a Diet of Human Milk and Bovine Milk-Based Products. J. Pediatr. 2010, 156, 562–567.e561. [Google Scholar] [CrossRef] [PubMed]
- Wickes, J.G. A history of infant feeding. IV. Nineteenth century continued. Arch. Dis. Child. 1953, 28, 416–422. [Google Scholar] [CrossRef]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Pammi, M.; Suresh, G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst. Rev. 2019, 11, CD013145. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.-Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the Establishment and Operation of Human Milk Banks in Europe: A Consensus Statement from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Unger, S. Human milk banking. Paediatr. Child Health 2010, 15, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.R.; Becker, A.; Fox, J.; Horgan, M.; Moores, R.; Pardalos, J.; Pinheiro, J.; Stewart, D.; Robinson, T. Implementing an exclusive human milk diet for preterm infants: Real-world experience in diverse NICUs. BMC Pediatr. 2023, 23, 237. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Hay, J.W.; Kim, J.H. Costs of Necrotizing Enterocolitis and Cost-Effectiveness of Exclusively Human Milk-Based Products in Feeding Extremely Premature Infants. Breastfeed. Med. 2011, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dziak, J.J.; Dierker, L.C.; Abar, B. The interpretation of statistical power after the data have been gathered. Curr. Psychol. 2020, 39, 870–877. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A. Prevention of Bronchopulmonary Dysplasia: A Summary of Evidence-Based Strategies. NeoReviews 2019, 20, e189–e201. [Google Scholar] [CrossRef]
- Soltys, F.; Philpott-Streiff, S.E.; Fuzzell, L.; Politi, M.C. The importance of shared decision-making in the neonatal intensive care unit. J. Perinatol. 2020, 40, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Cummings, C. Historical Perspectives: Shared Decision Making in the NICU. NeoReviews 2020, 21, e217–e225. [Google Scholar] [CrossRef]
- Modi, N. Repeating the errors of the past: The hazards of a commercial human milk industry. Pediatr. Res. 2024, 1–3. [Google Scholar] [CrossRef]
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update-III-Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ. J. 2022, 15, 100668. [Google Scholar] [CrossRef]
- Lokossou, Y.U.A.; Tambe, A.B.; Azandjèmè, C.; Mbhenyane, X. Socio-cultural beliefs influence feeding practices of mothers and their children in Grand Popo, Benin. J. Health Popul. Nutr. 2021, 40, 33. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R. Short-Term Trials and Long-Term Effects. Science 2001, 293, 2392–2394. [Google Scholar] [CrossRef] [PubMed]



| Study ID | Study Type | Sample Size (HMF-HM vs. HMF-BM) | GA(wks) or BW (g) | Primary Outcomes | Secondary Outcomes | Formulation HMF and BMF | Fortification Started at | End of the Intervention | Confounding Factors |
|---|---|---|---|---|---|---|---|---|---|
| Jensen, 2023 [14] Sweden | RCT | 115 vs. 113 | <28 wk | Composite of NEC stage II-III, Culture proven sepsis and mortality | NEC, death, sepsis, BPD, ROP, PVL, intensive care days, mechanical ventilation days, feeding intolerance | Humavant + 6, Prolacta vs. BMF of the responsible unit | 100 mL/kg/day | 34 wks PMA | NI |
| O’Connor, 2018 [15] Canada | RCT | 64 vs. 63 | <1250 g | Feeding interruption | Other measures of feeding tolerance, a dichotomous mortality and morbidity index (death, LOS, BPD, ROP, or NEC), fecal calprotectin, growth | Prolact +4/Prolact +6/Prolact +8, Prolacta vs. Similac Human Milk Fortifier Powder, Abbott Nutrition | 100 mL/kg/day | Whichever came first: 84 d of age/discharge/≥2 complete oral feeds daily over 3d | GA |
| Subgroup analysis of NCT00506584 [Sullivan trial] from Lucas et al., 2020 [16] USA, Austria | RCT | 82 vs. 32 | <1250 g | NEC | BPD, requirement for mechanical ventilation, ROP, sepsis, growth | Prolact + H2MF, Prolacta vs. Enfamil, Mead Johnson or Similac, Abbott Nutrition | 40 or 100 mL/kg/day for HMF and 100 mL/kg/day for BMF | Whichever came first: 91d of age/ discharge/≥4 complete oral feeds/day | GA |
| Subgroup analysis of NCT00506584 [Assad trial] from Lucas et al., 2020 [17] USA | Retrospective cohort | 87 vs. 127 | <28 wk or <1500 g | Length of time to full feds, length of stay, incidence of feeding tolerance | NEC, costs | Prolact + H2MF, Prolacta vs. Similac, Abbott Nutrition | 120–150 mL/kg/day | Discharge | GA, BW |
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No. of Participants (Studies) | Certainty of the Evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with HMF-BM | Risk with HMF-HM | ||||
| Mortality | 10 per 100 | 5 per 100 (3 to 10) | RR 0.50 (0.25 to 0.97) | 467 (3 RCTs) | ⨁◯◯◯ Very low a |
| NEC stage ≥ 2 | 8 per 100 | 5 per 100 (2 to 12) | RR 0.61 (0.27 to 1.42) | 467 (3 RCTs) | ⨁⨁◯◯ Low b |
| BPD at 36 weeks PMA | 49 per 100 | 42 per 100 (34 to 51) | RR 0.86 (0.70 to 1.04) | 449 (3 RCTs) | ⨁◯◯◯ Very low c |
| Retinopathy of Prematurity (any) | 17 per 100 | 14 per 100 (5 to 35) | RR 0.84 (0.33 to 2.13) | 457 (3 RCTs) | ⨁◯◯◯ Very low d |
| Late-onset Sepsis | 23 per 100 | 23 per 100 (14 to 39) | RR 1.01 (0.60 to 1.71) | 467 (3 RCTs) | ⨁◯◯◯ Very low e |
| Summary of findings (SoF) table | |||||
| HMF-HM compared to HMF-BM in Extreme Preterm Outcomes | |||||
| Patient or population: Extreme preterm or VLBW infants Setting: Neonatal Intensive Care Unit Intervention: HMF-HM Comparison: HMF-BM | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galis, R.; Trif, P.; Mudura, D.; Mazela, J.; Daly, M.C.; Kramer, B.W.; Diggikar, S. Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants—A Meta-Analysis. Nutrients 2024, 16, 910. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060910
Galis R, Trif P, Mudura D, Mazela J, Daly MC, Kramer BW, Diggikar S. Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants—A Meta-Analysis. Nutrients. 2024; 16(6):910. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060910
Chicago/Turabian StyleGalis, Radu, Paula Trif, Diana Mudura, Jan Mazela, Mandy C. Daly, Boris W. Kramer, and Shivashankar Diggikar. 2024. "Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants—A Meta-Analysis" Nutrients 16, no. 6: 910. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16060910