Development and Feasibility of an eHealth Diabetes Prevention Program Adapted for Older Adults—Results from a Randomized Control Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adaptations to the DPP-GLB
2.1.1. Supplemental Pedagogical and Behavior Change Theories for the Modification
2.1.2. Adaptation of Program Goals and Exercise, Diet, and Weight Recommendations for Older Adults
2.1.3. Changes to Implementation and Self-Monitoring That Support Program Fidelity and Remote Delivery
2.1.4. Participant and Interventionist Interactions and Communication in the Adaptation
2.2. Pilot Randomized Controlled Study Using the Adapted Intervention
2.3. Statistical Analysis
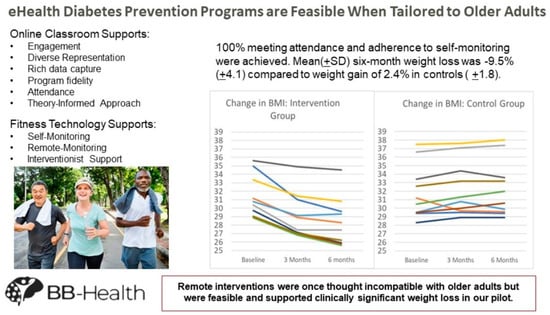
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federal Interagency Forum on Aging-Related Statistics. Older Americans 2020: Key Indicators of Well-Being; Forum, F.I., Ed.; Government Printing Office: Washington, DC, USA, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Prediabetes among Adults; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002, 25, 2165–2171. [Google Scholar] [CrossRef]
- Skrine Jeffers, K.; Castellon-Lopez, Y.; Grotts, J.; Mangione, C.M.; Moin, T.; Tseng, C.-H.; Turk, N.; Frosch, D.L.; Norris, K.C.; Duke, C.C.; et al. Diabetes Prevention Program attendance is associated with improved patient activation: Results from the Prediabetes Informed Decisions and Education (PRIDE) study. Prev. Med. Rep. 2019, 16, 100961. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Absetz, P.; Oldroyd, J.; Pronk, N.P.; Oldenburg, B. A systematic review of real-world diabetes prevention programs: Learnings from the last 15 years. Implement. Sci. 2015, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, A.J.; Bodicoat, D.H.; Greaves, C.J.; Russell, C.; Yates, T.; Davies, M.J.; Khunti, K. Diabetes prevention in the real world: Effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: A systematic review and meta-analysis. Diabetes Care 2014, 37, 922–933. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J. Am. Coll. Cardiol. 2014, 63 Pt B, 2985–3023. [Google Scholar] [CrossRef]
- Joiner, K.L.; Nam, S.; Whittemore, R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev. Med. 2017, 100, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, A.; Gilhooly, C.H.; Bukhari, A.; Das, S.K.; Martin, E.; Hatch, A.M.; Silver, R.E.; Montain, S.J.; Roberts, S.B. Development of a Videoconference-Adapted Version of the Community Diabetes Prevention Program, and Comparison of Weight Loss with In-Person Program Delivery. Mil. Med. 2019, 184, 647–652. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Delivering from a Distance: Reaching People at Home; Diabetes Prevention Program (DPP) Research Group, Ed.; CDC: Atlanta, GA, USA, 2020.
- Jahangiry, L.; Farhangi, M.A. Obesity paradigm and web-based weight loss programs: An updated systematic review and meta-analysis of randomized controlled trials. J. Health Popul. Nutr. 2021, 40, 16. [Google Scholar] [CrossRef]
- Shi, Y.; Wakaba, K.; Kiyohara, K.; Hayashi, F.; Tsushita, K.; Nakata, Y. Effectiveness and Components of Web-Based Interventions on Weight Changes in Adults Who Were Overweight and Obese: A Systematic Review with Meta-Analyses. Nutrients 2023, 15, 179. [Google Scholar] [CrossRef]
- Moin, T.; Damschroder, L.J.; AuYoung, M.; Maciejewski, M.L.; Havens, K.; Ertl, K.; Vasti, E.; Weinreb, J.E.; Steinle, N.I.; Billington, C.J.; et al. Results from a Trial of an Online Diabetes Prevention Program Intervention. Am. J. Prev. Med. 2018, 55, 583–591. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Gay, C.L.; Joiner, K.L.; Vittinghoff, E. A Novel Diabetes Prevention Intervention Using a Mobile App: A Randomized Controlled Trial with Overweight Adults at Risk. Am. J. Prev. Med. 2015, 49, 223–237. [Google Scholar] [CrossRef]
- Choi, N.G.; DiNitto, D.M.; Marti, C.N.; Choi, B.Y. Telehealth Use among Older Adults during COVID-19: Associations with Sociodemographic and Health Characteristics, Technology Device Ownership, and Technology Learning. J. Appl. Gerontol. 2021, 41, 600–609. [Google Scholar] [CrossRef]
- Qin, W. Technology Learning and the Adoption of Telehealth among Community-Dwelling Older Adults during the COVID-19 Outbreak. J. Appl. Gerontol. 2022, 41, 1651–1656. [Google Scholar] [CrossRef]
- Molin, F.; Haelermans, C.; Cabus, S.; Groot, W. Do feedback strategies improve students’ learning gain?-Results of a randomized experiment using polling technology in physics classrooms. Comput. Educ. 2021, 175, 104339. [Google Scholar] [CrossRef]
- Brickwood, K.J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, K.J.; Ahuja, K.D.K.; Watson, G.; O’Brien, J.A.; Williams, A.D. Effects of Activity Tracker Use with Health Professional Support or Telephone Counseling on Maintenance of Physical Activity and Health Outcomes in Older Adults: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e18686. [Google Scholar] [CrossRef] [PubMed]
- Ringeval, M.; Wagner, G.; Denford, J.; Paré, G.; Kitsiou, S. Fitbit-Based Interventions for Healthy Lifestyle Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e23954. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, C.; Yasuda, S.; Kita, Y. Interaction during online classes fosters engagement with learning and self-directed study both in the first and second years of the COVID-19 pandemic. Comput. Educ. 2023, 200, 104795. [Google Scholar] [CrossRef]
- Kumtepe, A.; Atasoy, E.; Kaya, Ö.; Şişman Uğur, S.; Dinçer, G.D.; Aydin, C.; Erdoğdu, E. An Interaction Framework for Open and Distance Learning: Learning Outcomes, Motivation, Satisfaction, and Perception. Online Acad. J. Inf. Technol. 2019, 10, 7–26. [Google Scholar] [CrossRef]
- Moore, R.L.; Miller, C.N. Fostering Cognitive Presence in Online Courses: A Systematic Review (2008–2020). Online Learn. 2022, 26, 130–149. [Google Scholar] [CrossRef]
- Hawkes, R.E.; Miles, L.M.; French, D.P. The theoretical basis of a nationally implemented type 2 diabetes prevention programme: How is the programme expected to produce changes in behaviour? Int. J. Behav. Nutr. Phys. Act. 2021, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T. Getting the Mix Right Again: An Updated and Theoretical Rationale for Interaction. Int. Rev. Res. Open Distrib. Learn. 2003, 4. [Google Scholar] [CrossRef]
- Fiock, H. Designing a Community of Inquiry in Online Courses. Int. Rev. Res. Open Distrib. Learn. 2020, 21, 135–153. [Google Scholar] [CrossRef]
- Garrison, D. Online community of inquiry review: Social, cognitive, and teaching presence issues. J. Asynchronous Learn. Netw. 2007, 11, 61–72. [Google Scholar] [CrossRef]
- Garrison, D.R.; Arbaugh, J.B. Researching the community of inquiry framework: Review, issues, and future directions. Internet High. Educ. 2007, 10, 157–172. [Google Scholar] [CrossRef]
- Christie, D.; Channon, S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: A clinical review. Diabetes Obes. Metab. 2014, 16, 381–387. [Google Scholar] [CrossRef]
- Dineen, T.E.; Bean, C.; Jung, M.E. Successes and Challenges From a Motivational Interviewing-Informed Diabetes Prevention Program Situated in the Community. Health Promot. Pract. 2022, 25, 274–284. [Google Scholar] [CrossRef]
- Jackson, R.; Asimakopoulou, K.; Scammell, A. Assessment of the transtheoretical model as used by dietitians in promoting physical activity in people with type 2 diabetes. J. Hum. Nutr. Diet. 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change, 3rd ed.; Guilford Press: New York, NY, USA, 2013; pp. xii–482. [Google Scholar]
- Jiménez-Zazo, F.; Romero-Blanco, C.; Castro-Lemus, N.; Dorado-Suárez, A.; Aznar, S. Transtheoretical Model for Physical Activity in Older Adults: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9262. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, D.E.; Kim, K.; Shim, J.E.; Sung, E.; Kang, J.H.; Hwang, J.Y. Development of tailored nutrition information messages based on the transtheoretical model for smartphone application of an obesity prevention and management program for elementary-school students. Nutr. Res. Pract. 2017, 11, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, H.A.; Min, Y.H. Transtheoretical Model-based Nursing Intervention on Lifestyle Change: A Review Focused on Intervention Delivery Methods. Asian Nurs. Res. 2015, 9, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.; Mingoti, S.A.; Cardoso, C.S.; Mendonça Rde, D.; Lopes, A.C. Intervention based on Transtheoretical Model promotes anthropometric and nutritional improvements—A randomized controlled trial. Eat. Behav. 2015, 17, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-X.; Ho, S.-C.; Sit, J.W.H.; He, H.-G. Effect of a Transtheoretical Model–Based Stage-Matched Exercise Intervention on Exercise Behavior and Angina in Patients with Coronary Heart Disease: A Randomized Controlled Trial. J. Cardiovasc. Nurs. 2014, 59, 384–392. [Google Scholar]
- AuYoung, M.; Moin, T.; Richardson, C.R.; Damschroder, L.J. The Diabetes Prevention Program for Underserved Populations: A Brief Review of Strategies in the Real World. Diabetes Spectr. 2019, 32, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Office of Minority Health. Diabetes Prevention Programs: Equity Tailored Resources; The Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2023. [Google Scholar]
- Beasley, J.M.; Kirshner, L.; Wylie-Rosett, J.; Sevick, M.A.; DeLuca, L.; Chodosh, J. BRInging the Diabetes prevention program to GEriatric populations (BRIDGE): A feasibility study. Pilot. Feasibility Stud. 2019, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, J.; Gaillard, T.; Caceres, S.; Hollifield, M.; Huffman, F. Barriers to Participating in Diabetes Care Behaviors in Hard to Reach Older Hispanics. Nurs. Prim. Care 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Realmuto, L.; Weiss, L.; Walker, E. Struggling to Stay on Track: Participants Share Benefits and Barriers to Completing the National Diabetes Prevention Program; New York Academy of Medicine: New York, NY, USA, 2017. [Google Scholar]
- Gelles-Watnick, R. Americans’ Use of Mobile Technology and Home Broadband; Pew Research: Washington, DC, USA, 2024. [Google Scholar]
- Kramer, M.K.; Kriska, A.M.; Venditti, E.M.; Miller, R.G.; Brooks, M.M.; Burke, L.E.; Siminerio, L.M.; Solano, F.X.; Orchard, T.J. Translating the Diabetes Prevention Program: A Comprehensive Model for Prevention Training and Program Delivery. Am. J. Prev. Med. 2009, 37, 505–511. [Google Scholar] [CrossRef]
- Prochaska, J.; DiClemente, C. Coping and Substance Use; Academic: New York, NY, USA, 1985. [Google Scholar]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change. Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, L.P.; De la Roca-Chiapas, J.M.; Colunga-Rodriguez, C.; Preciado-Serrano, M.L.; Daneri-Navarro, A.; Pedroza-Cabrera, F.J.; Martinez-Arriaga, R.J. Validation of Health Behavior and Stages of Change Questionnaire. Breast Cancer 2017, 9, 199–205. [Google Scholar] [CrossRef]
- van der Veen, J.; Bakx, C.; van den Hoogen, H.; Verheijden, M.; van den Bosch, W.; van Weel, C.; van Staveren, W. Stage-matched nutrition guidance for patients at elevated risk for cardiovascular disease: A randomized intervention study in family practice. J. Fam. Pract. 2002, 51, 751–758. [Google Scholar] [PubMed]
- Johnson, S.S.; Driskell, M.M.; Johnson, J.L.; Dyment, S.J.; Prochaska, J.O.; Prochaska, J.M.; Bourne, L. Transtheoretical model intervention for adherence to lipid-lowering drugs. Dis. Manag. 2006, 9, 102–114. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Redding, C.A.; Evers, K.E. The transtheoretical model and stages of change. In Health Behavior and Health Education: Theory, Research, and Practice, 4th ed.; Jossey-Bass: San Francisco, CA, USA, 2008; pp. 97–121. [Google Scholar]
- Prochaska, J.O.; Velicer, W.F.; Redding, C.; Rossi, J.S.; Goldstein, M.; DePue, J.; Greene, G.W.; Rossi, S.R.; Sun, X.; Fava, J.L.; et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Prev. Med. 2005, 41, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.; Langley-Evans, S.C.; Harrington, M.; Swift, J.A. Setting targets leads to greater long-term weight losses and ‘unrealistic’ targets increase the effect in a large community-based commercial weight management group. J. Hum. Nutr. Diet. 2016, 29, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Nothwehr, F.; Yang, J. Goal setting frequency and the use of behavioral strategies related to diet and physical activity. Health Educ. Res. 2007, 22, 532–538. [Google Scholar] [CrossRef]
- Rishor-Olney, C.R.; Hinson, M.R. Mediterranean Diet; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary Fiber and Weight Regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Nowson, C.; O’Connell, S. Protein Requirements and Recommendations for Older People: A Review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef]
- Kraun, L.; De Vliegher, K.; Ellen, M.; van Achterberg, T. Interventions for the empowerment of older people and informal caregivers in transitional care decision-making: Short report of a systematic review. BMC Geriatr. 2023, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dong, H. Design Empowering Active Aging: A Resource-Based Design Toolkit. Int. J. Hum. Comput. Interact. 2023, 39, 601–611. [Google Scholar] [CrossRef]
- An, M.; Dusing, S.C.; Harbourne, R.T.; Sheridan, S.M.; Consortium, S.T.-P. What Really Works in Intervention? Using Fidelity Measures to Support Optimal Outcomes. Phys. Ther. 2020, 100, 757–765. [Google Scholar] [CrossRef]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and facilitators to the use of e-health by older adults: A scoping review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef]
- Ufholz, K. Peer Support Groups for Weight Loss. Curr. Cardiovasc. Risk Rep. 2020, 14, 19. [Google Scholar] [CrossRef]
- Venditti, E.M.; Kramer, M.K. Necessary components for lifestyle modification interventions to reduce diabetes risk. Curr. Diabetes Rep. 2012, 12, 138–146. [Google Scholar] [CrossRef]
- Caso, G.; Vecchio, R. Factors influencing independent older adults (un)healthy food choices: A systematic review and research agenda. Food Res. Int. 2022, 158, 111476. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Khamseh, F.; Ebadi, A.; Hajiamini, Z.; Navidian, A. Comparison of group motivational interviewing and multimedia education on elderly lifestyle. J. Educ. Health Promot. 2018, 7, 133. [Google Scholar] [CrossRef]
- Silver, R.E.; Gerber, S.; Das, S.K.; Greene, S.; Dao, M.C.; Sunwoo, J.; Ceglia, L.; Dix, S.R.; Barger, K.; Franceschini, M.A.; et al. Protocol for the Nutrition for Brain and Body Health Study: A Randomized Feasibility Trial. PLoS ONE, 2023; submitted. [Google Scholar]
- Silver, R.; Gerber, S.; Das, S.; Dao, M.; Ceglia, L.; Cohen, R.; Franceschini, M.; Roberts, S. P23-071-23 BB-Health: A Pilot Randomized Feasibility Trial of Food Supplement and Behavioral Weight Management Interventions to Prevent Cognitive Decline in Older Adults. Curr. Dev. Nutr. 2023, 7, 100180. [Google Scholar]
- Gerber, S.; Silver, R.; Das, S.K.; Dao, M.C.; Ceglia, L.; Morcos, C.; Ramirez, I.; Roberts, S. P23-030-23 Development and Feasibility of a Remotely Delivered Healthy Lifestyle Intervention for Older Adults. Curr. Dev. Nutr. 2023, 7, 100142. [Google Scholar] [CrossRef]
- Kramer, M.K.; Vanderwood, K.K.; Arena, V.C.; Miller, R.G.; Meehan, R.; Eaglehouse, Y.L.; Schafer, G.; Venditti, E.M.; Kriska, A.M. Evaluation of a Diabetes Prevention Program Lifestyle Intervention in Older Adults: A Randomized Controlled Study in Three Senior/Community Centers of Varying Socioeconomic Status. Diabetes Educ. 2018, 44, 118–129. [Google Scholar] [CrossRef]
- Horn, D.B.; Almandoz, J.P.; Look, M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad. Med. 2022, 134, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, K.R.; Pignol, A.E.; Holm, J.E.; Vogeltanz-Holm, N. Telephone-based motivational interviewing to promote physical activity and stage of change progression in older adults. J. Aging Phys. Act. 2014, 22, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.K.; Simpson, K.; Lloyd, B.; Bauman, A.E.; Singh, M.A. Behavioral strategies in diabetes prevention programs: A systematic review of randomized controlled trials. Diabetes Res. Clin. Pract. 2011, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program (DPP) Research Group. Long-Term Safety, Tolerability, and Weight Loss Associated with Metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Sarvary, M.A.; Gifford, K.M. The Benefits of a Real-Time Web-Based Response System for Enhancing Engaged Learning in Classrooms and Public Science Events. J. Undergrad. Neurosci. Educ. 2017, 15, E13–E16. [Google Scholar]
- Johnston, B.; Ruffalo, L.; Nelson, D.; O’Connor, S.; Young, S. Foundations of Community Engagement: A Series for Effective Community-Engaged Research. MedEdPORTAL 2023, 19, 11350. [Google Scholar] [CrossRef]
- Seifert, A.; Reinwand, D.A.; Schlomann, A. Designing and Using Digital Mental Health Interventions for Older Adults: Being Aware of Digital Inequality. Front. Psychiatry 2019, 10, 463542. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Katthula, V.; Moustakas, E. Too old for technology? Use of wearable healthcare devices by older adults and their willingness to share health data with providers. Health Inform. J. 2021, 27, 14604582211058073. [Google Scholar] [CrossRef]
- Farivar, S.; Abouzahra, M.; Ghasemaghaei, M. Wearable device adoption among older adults: A mixed-methods study. Int. J. Inf. Manag. 2020, 55, 102209. [Google Scholar] [CrossRef]
- Moore, K.; O’Shea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamäki, A.; Condell, J.; Nordström, A.; et al. Older Adults’ Experiences with Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR Mhealth Uhealth 2021, 9, e23832. [Google Scholar] [CrossRef]
- Roberts, M.; Sappington, E.; Yalcin, A.; Lowenkron, J.; Amzallag, D.; VandeWeerd, C. Increasing Adoption and Utility of Smartwatches in Older Adults. Innov. Aging 2020, 4 (Suppl. S1), 411–412. [Google Scholar] [CrossRef]
- Kim, S.; Choudhury, A. Comparison of Older and Younger Adults’ Attitudes toward the Adoption and Use of Activity Trackers. JMIR Mhealth Uhealth 2020, 8, e18312. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Gao, X.; Chen, M.; Van Dam, R.M. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Progress. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]



| Baseline Characteristics | Intervention n = 10 | Control n = 10 |
|---|---|---|
| Age, years mean(SD) | 65.5(4.6) | 65.3(6.3) |
| BMI, kg/m2 mean(SD) | 31.3(2.63) | 31.3(2.98) |
| Female, n(%) | 7(70) | 2(20) |
| Race n(%) | ||
| Black/African American ƚ | 4(40) | 1(10) |
| Hispanic/Latine | 1(10) | 1(10) |
| White ͊ | 5(50) | 8(80) |
| Educational Attainment n(%) | ||
| High School Diploma/Equivalent | 2(20) | 3(30) |
| Associate’s Degree | 2(20) | 0(0) |
| Bachelor’s Degree | 6(60) | 7(70) |
| Weight Category n(%) | ||
| 25 < BMI < 30 | 4(40) | 4(40) |
| BMI ≥ 30 | 6(60) | 6(60) |
| BB-Health Adaptation | DPP-GLB |
|---|---|
Technology:
|
|
Online classroom materials:
|
|
Affirmations and reports:
|
|
Flexible diet composition:
|
|
Meetings:
|
|
Goal setting successively rather than fixed:
|
|
Theory-informed changes
|
|
| Intervention (n = 10) | ||||||
|---|---|---|---|---|---|---|
| 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | |
| Adherence | ||||||
| Daily kcal, mean(SD) | 1284(305) | 1289(352) | 1289(387) | 1407(742) | 1252(466) | 1326(490) |
| Calorie target, %(SD) | 75(25) | 77(27) | 75(32) | 70(28) | 74(25) | 73(29) |
| Steps/day, mean(SD) | 10,918(6020) | 10,765(5871) | 10,872(5738) | 10,932(5790) | 11,453(5709) | 11,608(5946) |
| Step target, %(SD) | 95(11) | 93(17) | 93(17) | 90(24) | 85(27) | 88(21) |
| MVPA/week, mean(SD) | 455(436) | 474(459) | 492(430) | 498(418) | 530(395) | 533(395) |
| Exercise target, %(SD) | 70(42) | 68(44) | 73(34) | 63(41) | 65(43) | 70(44) |
| Attendance, %(SD) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) |
| Scale weight, %(SD) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) |
| Food logging, %(SD) | 100(0) | 100(0) | 100(0) | 100(0) | 98(3) | 100(0) |
| Engagement, %(SD) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) | 100(0) |
| Outcomes | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months |
| Weight change, %(SD) | −3.8(1.8) | −5.5(3.4) | −7.0(3.0) | −7.5(3.6) | −9.0 (4.4) | −9.5(4.1) |
| BMI, kg/m2 mean(SD) | 29.9(2.6) | 29.4(2.6) | 29.1(2.7) | 28.73(2.8) | 28.40(2.8) | 28.3(2.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, S.; Silver, R.E.; Das, S.K.; Greene, S.S.; Dix, S.R.; Ramirez, I.; Morcos, C.L.; Dao, M.C.; Ceglia, L.; Roberts, S.B. Development and Feasibility of an eHealth Diabetes Prevention Program Adapted for Older Adults—Results from a Randomized Control Pilot Study. Nutrients 2024, 16, 930. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16070930
Gerber S, Silver RE, Das SK, Greene SS, Dix SR, Ramirez I, Morcos CL, Dao MC, Ceglia L, Roberts SB. Development and Feasibility of an eHealth Diabetes Prevention Program Adapted for Older Adults—Results from a Randomized Control Pilot Study. Nutrients. 2024; 16(7):930. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16070930
Chicago/Turabian StyleGerber, Suzannah, Rachel E. Silver, Sai Krupa Das, Savana S. Greene, Sadie R. Dix, Isabella Ramirez, Christina L. Morcos, Maria Carlota Dao, Lisa Ceglia, and Susan B. Roberts. 2024. "Development and Feasibility of an eHealth Diabetes Prevention Program Adapted for Older Adults—Results from a Randomized Control Pilot Study" Nutrients 16, no. 7: 930. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16070930