Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit
Abstract
:1. Introduction
2. Results and Discussion
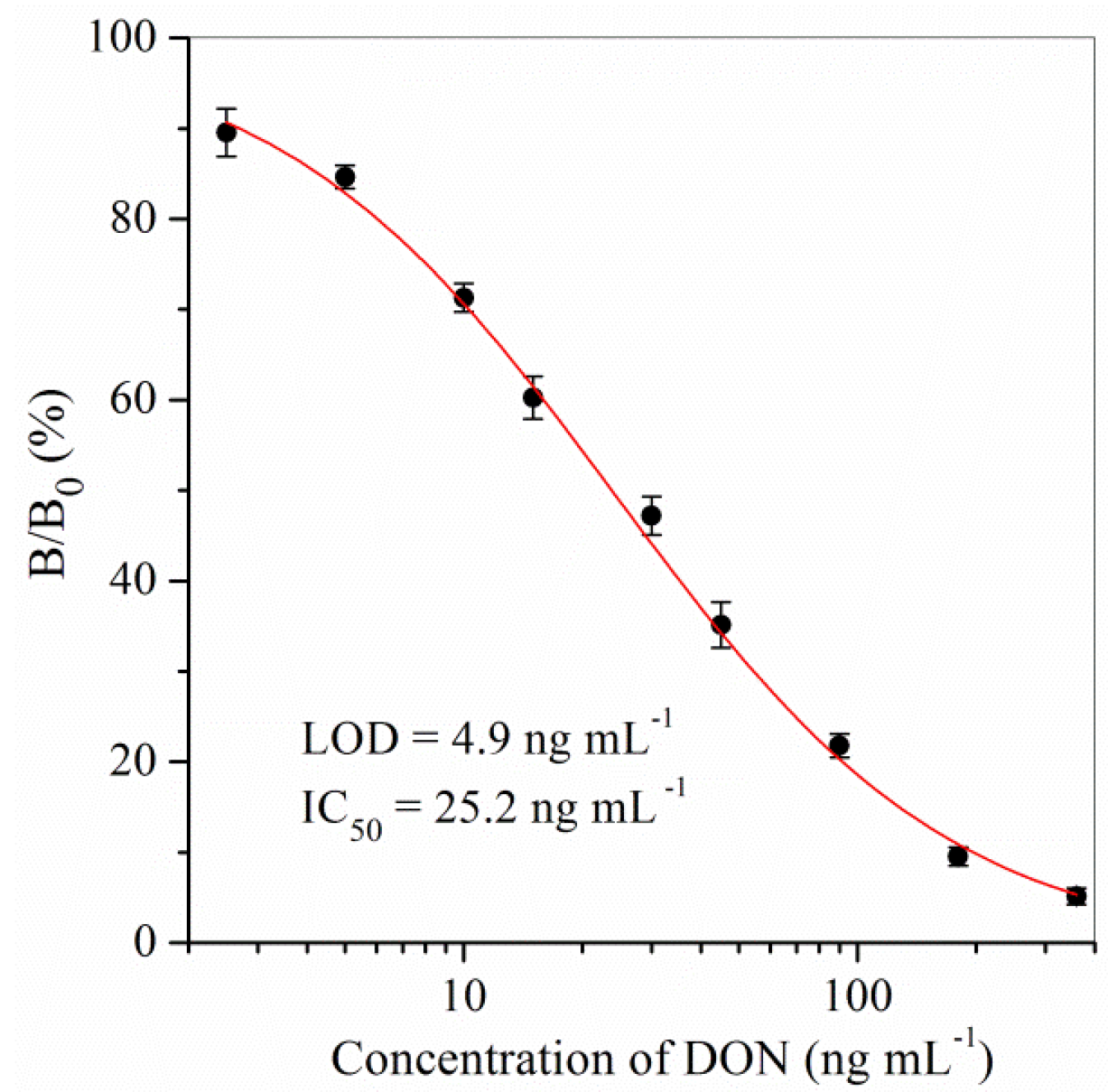
2.1. Sensitivity
2.2. Specificity
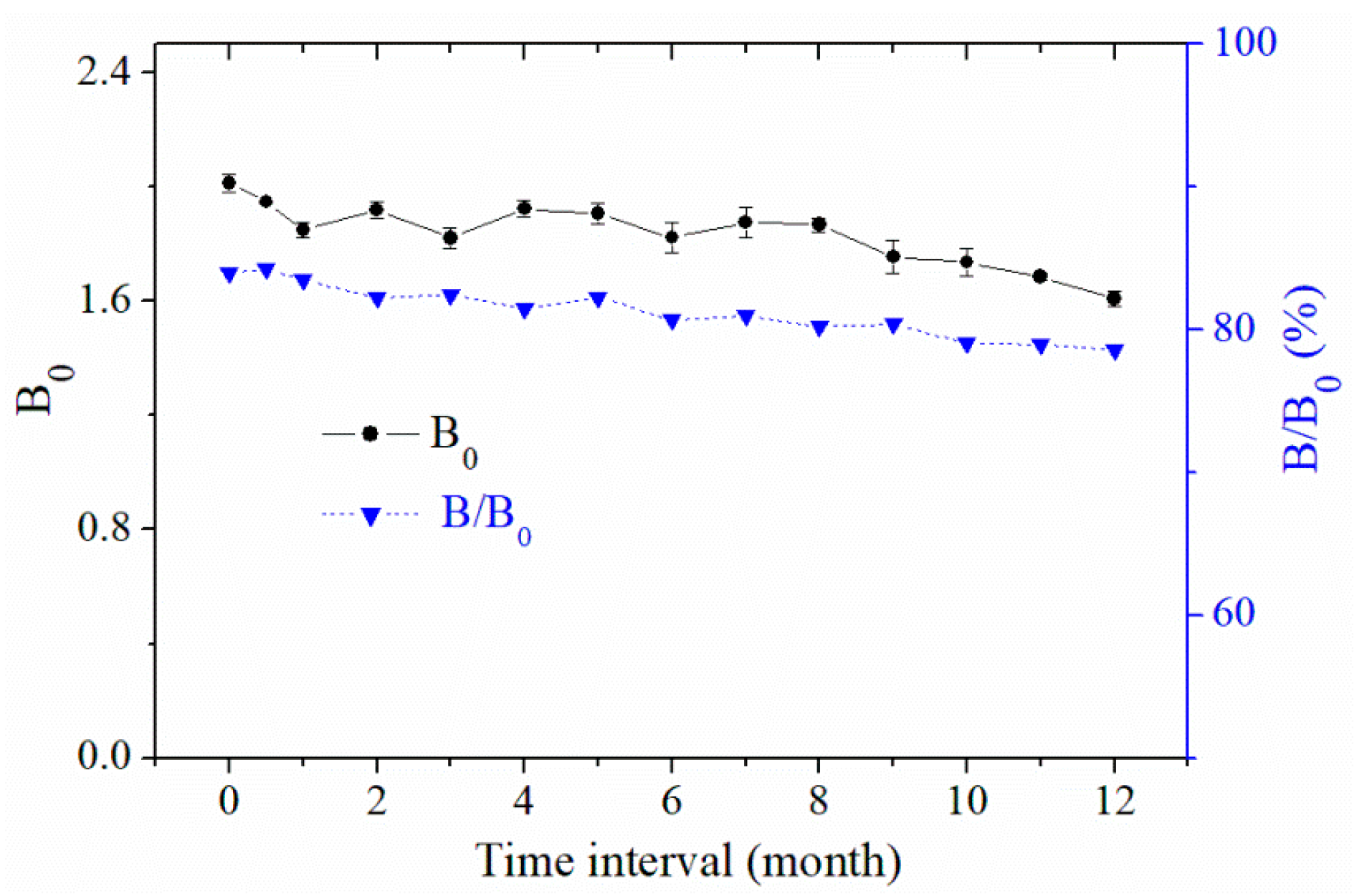
2.3. Accuracy and Precision
2.4. Determination and Evaluation of Authentic Samples
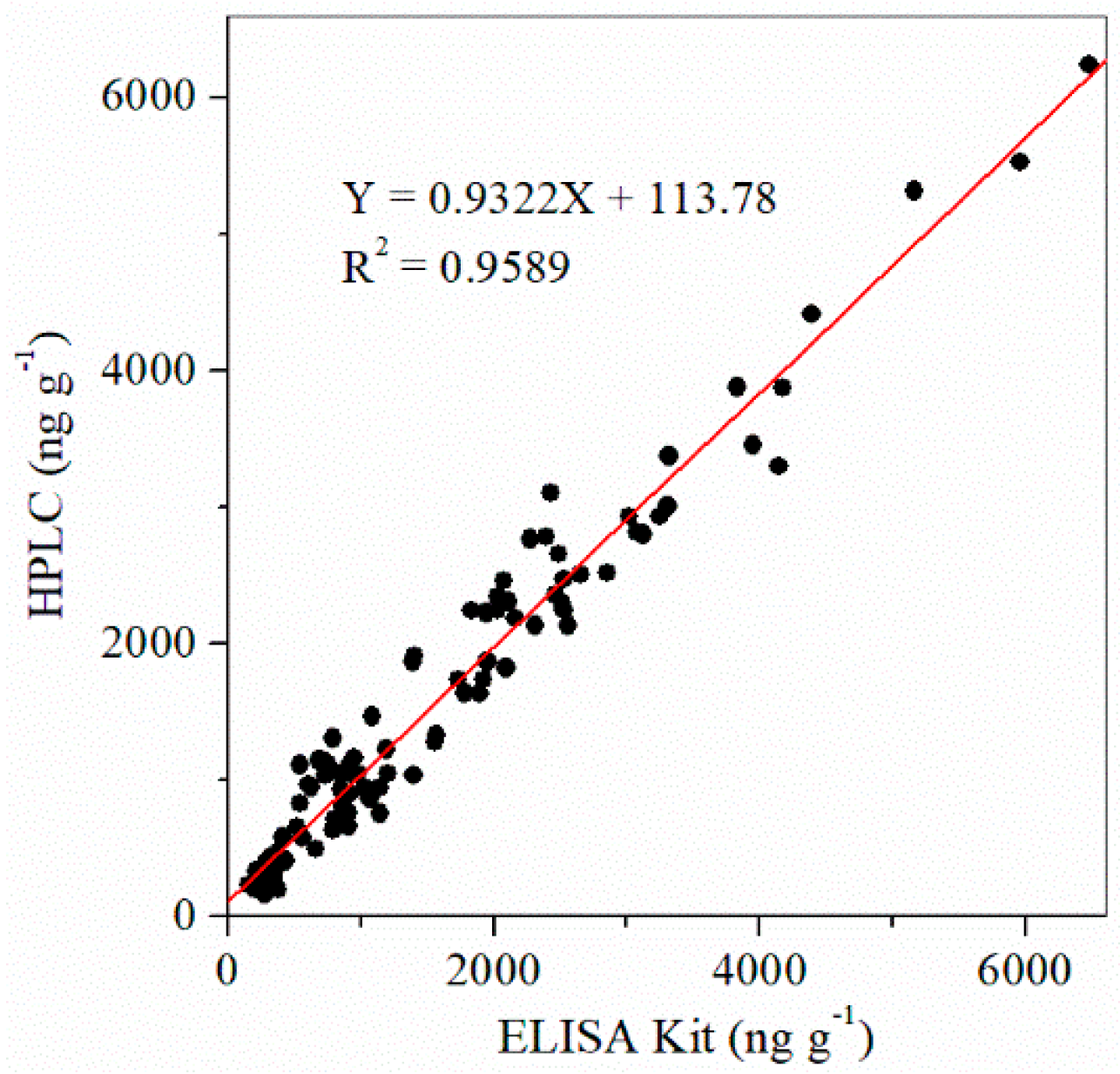
2.5. Correlation of ELISA Kit and HPLC
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation of Antigen and McAb
4.3. Development of the DON ELISA Kit
4.3.1. ELISA Kit Procedure
4.3.2. Standard Curve
4.3.3. ELISA Kit Parameters
4.3.4. CRs Experiment
4.4. Adjustment of ELISA Kit Conditions
4.5. Instructions for the ELISA Kit
4.6. Spiked Samples Analysis
4.7. Determination in Authentic Contaminated Samples
4.8. Confirmation of the ELISA Kit with HPLC
Author Contributions
Funding
Conflicts of Interest
References
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Li, H.; Xu, J.H.; Shi, J.R. Enzyme-linked immunosorbent-assay for deoxynivalenol (DON). Toxins 2011, 3, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.X.; Peng, Z.; Chen, L.K.; Nüssler, A.K.; Liu, L.G.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Kuzdralinski, A.; Solarska, E.; Muszynska, M. Deoxynivalenol and zearalenone occurence in beers analysed by an enzyme-linked immunosorbent assay method. Food Control 2013, 29, 22–24. [Google Scholar] [CrossRef]
- Righetti, L.; Galaverna, G.; Dall’Asta, C. Group detection of DON and its modified forms by an ELISA kit. Food Addit. Contam. A 2017, 34, 248–254. [Google Scholar] [CrossRef]
- Ji, J.; Zhu, P.; Cui, F.C.; Pi, F.W.; Zhang, Y.Z.; Li, Y.; Wang, J.S.; Sun, X.L. The antagonistic effect of mycotoxins deoxynivalenol and zearalenone on metabolic profiling in serum and liver of mice. Toxins 2017, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Fruhmann, P.; Hametner, C.; Adam, G.; Frohlich, J.; Krska, R. Direct quantification of deoxynivalenol glucuronide in human urine as biomarker of exposure to the Fusarium mycotoxin deoxynivalenol. Anal. Bioanal. Chem. 2011, 401, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hanschmann, G.; Krieg, D. Verhalten von mykotoxinen bei der ethanolerzeugung aus getreide. Mycotoxin Res. 2006, 22, 174–177. [Google Scholar] [CrossRef] [PubMed]
- China National Standard No. GB2761-2017; Ministry of Health of P. R. China: Beijing, China, 2017.
- Fernandez, C.; Stack, M.E.; Musser, S.M. Determination of deoxynivalenol in 1991 U.S. winter and spring wheat by high-performance thin-layer chromatography. J. AOAC 1994, 77, 628–630. [Google Scholar]
- Wang, X.Y. Determination of deoxynivalenol in maize by using gas chromatography-electron capture detector. J. Changzhi Univ. 2006, 23, 7–10. [Google Scholar]
- Wang, Z.P.; Wang, D.L.; Feng, Z.S.; Yang, J.; Wang, X.J.; Chen, X. Detection of deoxynivalenol in malting barley by immunoaffinity column clear up and high performance liquid chromatography. Food Ferment. Ind. 2008, 34, 137–139. [Google Scholar]
- Cohen, H.; Lapointe, M. Capillary gas-liquid chromatographic determination of vomitoxin in cereal grains. J. AOAC 1982, 65, 1429–1434. [Google Scholar]
- Li, W.C.; Shen, X.G.; Liu, R.; Zhang, J.H.; Yu, J.X.; Xie, Y.P.; Deng, X.B. Study on the determination of deoxynivalenol in edible tissues of swine by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. South Chian Agric. Univ. 2013, 34, 101–105. [Google Scholar]
- Zhang, Z.; Zhu, N.F.; Huang, M.L.; Liang, Y.R.; Zeng, K.; Wu, X.Y.; Liu, Z.J.; Ma, Q.C.; Qu, G.B.; Shi, J.B. Sensitive immunoassay for simultaneous determination of tetrabromobisphenol A bis(2-hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether: An effective and reliable strategy to estimate the typical tetrabromobisphenol A derivative and byproduct in aquatic environments. Environ. Pollut. 2017, 229, 431–438. [Google Scholar] [PubMed]
- Wang, S.T.; Gui, W.J.; Guo, Y.R.; Zhu, G.N. Preparation of a multi-hapten antigen and broad specificity polyclonal antibodies for a multiple pesticide immunoassay. Anal. Chim. Acta 2007, 587, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.F.; Zhao, H.; Liu, T.C.; Hou, J.J.; Ren, Z.Q.; Wu, Y.S.; Dong, W.Q.; Huang, W.H. Simultaneous determination of the cytokeratin 19 fragment and carcinoembryonic antigen in human serum by magnetic nanoparticle-based dual-label time-resolved fluoroimmunoassay. RSC Adv. 2014, 4, 55229–55236. [Google Scholar] [CrossRef]
- Jin, M.J.; Shao, H.; Jin, F.; Gui, W.J.; Shi, X.M.; Wang, J.; Zhu, G.N. Enhanced competitive chemiluminescent enzyme immunoassay for the trace detection of insecticide triazophos. J. Food Sci. 2012, 77, T99–T104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Li, C.S.; Wu, M.; Zhang, Y.; Ma, X.F.; Cheng, H.; Yan, J.H. Development of an ultrasensitive immunochromatographic assay (ICA) strip for the rapid detection of phenylethanolamine A in urine and pork samples. J. Food Sci. 2015, 80, T894–T899. [Google Scholar]
- Zhu, F.; Mao, C.M.; Du, D.L. Time-resolved immunoassay based on magnetic particles for the detection of diethyl phthalate in environmental water samples. Sci. Total Environ. 2017, 601, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Liu, C.; Luo, X.Y.; Ji, R.; Ran, L.; Chang, D.; Chang, J. Determination and study of deoxynivalenol (DON) in wheat using indirect competitive enzyme-linked immunosorbent assay based on monoclonal antibody. Acta Microbiol. Sin. 1994, 31, 65–70. [Google Scholar]
- Maragos, C.M.; McCormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immunol. 2000, 12, 181–192. [Google Scholar] [CrossRef]
- Santos, J.S.D.; Takabayashi, C.R.; Ono, E.Y.S.; Itano, E.N.; Mallmann, C.A.; Kawamura, O.; Hirooka, E.Y. Immunoassay based on monoclonal antibodies versus LC-MS: deoxynivalenol in wheat and flour in Southern Brazil. Food Addit. Contam. A 2011, 28, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Zhou, Y.J.; Zhou, M.G. A sensitive chemiluminescence enzyme immunoassay for the determination of deoxynivalenol in wheat samples. Anal. Methods 2015, 7, 2196–2202. [Google Scholar] [CrossRef]
- Li, C.L.; Wen, K. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016, 79, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Huang, B.; Li, Q.P.; Zhang, L.F.; Jin, J. Ultrasensitive time-resolved fluoroimmunoassay of deoxynivalenol. Food Sci. 2009, 3, 398–402. [Google Scholar]
- Zhang, J.; Gao, L.; Zhou, B. Simultaneous detection of deoxynivalenol and zearalenone by dual-label time-resolved fluorescence immunoassay. J. Sci. Food Agric. 2011, 91, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Z.P.; He, Q.H.; Deng, S.Z.; Li, L.S.; Li, Y.P. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chem. 2010, 119, 834–839. [Google Scholar] [CrossRef]
- Kadota, T.; Takezawa, Y.; Hirano, S.; Tajima, O.; Maragos, C.M.; Nakajima, T.; Tanaka, T.; Kamata, Y.; Sugita-Konishi, Y. Rapid detection of nivalenol and deoxynivalenol in wheat using surface plasmon resonance immunoassay. Anal. Chim. Acta 2010, 673, 173–178. [Google Scholar] [CrossRef]
- Schnerr, H.; Vogel, R.; Niessen, L. A biosensor-based immunoassay for rapid screening of deoxynivalenol contamination in wheat. Food Agric. Immunol. 2002, 14, 313–321. [Google Scholar] [CrossRef]
- Yu, Q.; Li, H.; Li, C.L.; Zhang, S.X.; Shen, J.Z.; Wang, Z.H. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Qiu, Y.L.; He, Q.H.; Xu, Y.; Bhunia, A.K.; Tu, Z.; Chen, B.; Liu, Y.Y. Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay. Anal. Chim. Acta 2015, 887, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Pagkali, V.; Petrou, P.S.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Moser, I.; Gajos, K.; Budkowski, A.; Economou, A.; et al. Simultaneous determination of aflatoxin B1, fumonisin B1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2018, 359, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.L.; Yi, H.; Dai, H.; Fang, D.D.; Hong, Z.S.; Lin, D.M.; Zheng, X.Q.; Lin, Y.Y. Fluoro-coumarin silicon phthalocyanine sensitized integrated electrochemiluminescence bioprobe constructed on TiO2 MOFs for the sensing of deoxynivalenol. Sensor. Actuators B-Chem. 2018, 269, 27–35. [Google Scholar] [CrossRef]
- Li, Y.S.; Shi, W.M.; Shen, J.Z.; Zhang, S.X.; Cheng, L.L.; Wang, Z.H. Development of a rapid competitive indirect ELISA procedure for the determination of deoxynivalenol in cereals. Food Agric. Immunol. 2012, 23, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Lee, H.M.; Song, S.O.; Cha, S.H.; Wee, S.B.; Bischoff, K.; Park, S.W.; Son, S.W.; Kang, H.G.; Cho, M.H. Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticle-based extraction and an enzyme-linked immunosorbent assay. J. Vet. Sci. 2013, 14, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmistrova, N.A.; Rusanova, T.Y.; Yurasov, N.A.; Goryacheva, I.Y.; Saeger, S.D. Multi-detection of mycotoxins by membrane based flow-through immunoassay. Food Control 2014, 46, 462–469. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Hua, X.D.; Li, M.; Yin, W.; Shi, H.Y.; Wang, M.H. Development of a sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) based on the monoclonal antibody for the detection of the benzothiostrobin. RSC Adv. 2014, 4, 24406–24411. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, B.; Sun, J.F.; Yi, Y.H.; Li, M.; Du, D.L.; Zhu, F.; Luan, J.W. Highly efficient detection of salbutamol in environmental water samples by an enzyme immunoassay. Sci. Total Environ. 2018, 613, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Wei, X.; Ren, K.W.; Zhu, G.B.; Zhang, Z.; Wang, J.G.; Du, D.L. Highly efficient detection of paclobutrazol in environmental water and soil samples by time-resolved fluoroimmunoassay. Sci. Total Environ. 2016, 569, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zou, Y.M.; Liu, J.X.; Wei, W.; Zhang, M.; Zhou, J.; Zhang, Z.; Gai, Z.K. Enzyme-linked immunosorbent assay for triclocarban in aquatic environments. Water Sci. Technol. 2015, 72, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- China National Standard No. GB 5009.111-2016; Ministry of Health of P. R. China: Beijing, China, 2016.

| Methods | Linear Range (ng mL−1) | LOD (ng mL−1) | IC50 (ng mL−1) | References |
|---|---|---|---|---|
| ELISA | 10–100,000 | 20 | 3407.7 | [2] |
| ELISA | 5–1000 | 5.0 | – | [21] |
| ELISA | – | 0.2 | 18 | [22] |
| ELISA | – | 6.1 | 61.1 | [35] |
| ELISA | – | – | 23.4 | [37] |
| CLEIA | 1.7–170.0 | 0.49 | 17 | [24] |
| FPIA | 447.5–3780 | 242.0 | 1300 | [25] |
| TRFIA | 0.01–100 | 0.01 | 4.84 | [26] |
| TRFIA | 0.0194–100 | 0.0194 | – | [27] |
| GICA | – | – | 50 | [28] |
| SPR-immunoassay | 130–10,000 | 2.5 | 720 | [30] |
| Silver staining GICA | – | – | 40 | [31] |
| Nanobody-based ELISA | 2.2–62.2 | 1.2 | 8.8 | [32] |
| Optical immunosensor | 2.5–125 | 2.5 | 24 | [33] |
| Electrochemiluminescence immunosensor | 0.0001–20 | 0.00003 | – | [34] |
| ELISA kit | 4.9–128.9 | 4.9 | 25.2 | This study |
| Compound | IC50 (ng mL−1) | CR (%) |
|---|---|---|
| DON | 25.2 | 100 |
| 3-AC-DON | 442.1 | 5.7 |
| 15-AC-DON | >5000 | <0.5 |
| DON-3-G | >10,000 | <0.3 |
| T-2 | >10,000 | <0.3 |
| OTA | >10,000 | <0.3 |
| ZEN | >10,000 | <0.3 |
| AFB1 | >10,000 | <0.3 |
| Sample | Spiked (ng g−1) | Mean Recovery ± SD (%) | RSD (%) | Sample | Spiked (ng g−1) | Mean Recovery ± SD (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Wheat flour | 200 | 87.1 ± 6.9 | 7.9 | Corn | 200 | 88.1 ± 5.7 | 6.5 |
| 500 | 95.0 ± 5.3 | 5.6 | 500 | 98.4 ± 7.3 | 7.4 | ||
| 1000 | 113.2 ± 9.3 | 8.2 | 1000 | 95.2 ± 6.2 | 6.5 | ||
| DDGS | 200 | 79.4 ± 7.5 | 9.4 | Wheat | 200 | 107.0 ± 7.6 | 7.1 |
| 500 | 89.2 ± 6.2 | 7.0 | 500 | 106.7 ± 13.5 | 12.6 | ||
| 1000 | 104.4 ± 5.8 | 5.5 | 1000 | 96.4 ± 6.3 | 6.5 | ||
| Feed | 200 | 77.1 ± 9.2 | 11.9 | Wheat bran | 200 | 76.3 ± 10.1 | 13.2 |
| 500 | 81.7 ± 5.6 | 6.8 | 500 | 95.0 ± 4.4 | 4.6 | ||
| 1000 | 89.4 ± 3.5 | 3.9 | 1000 | 96.5 ± 4.1 | 4.2 | ||
| Corn meal | 200 | 114.5 ± 7.8 | 6.8 | ||||
| 500 | 93.3 ± 3.9 | 4.2 | |||||
| 1000 | 103.7 ± 4.6 | 4.4 |
| Sample | Number | Average (ng g−1) | Range (ng g−1) | Detection Rate (%) | a Less than 200 ng g−1 (%) | 200–1000 ng g−1 (%) | 1000–3000 ng g−1 (%) | Above 3000 ng g−1 (%) |
|---|---|---|---|---|---|---|---|---|
| Wheat flour | 135 | 1482.4 | 203.3–5164.7 | 91.8 | 8.2 | 28.9 | 40.0 | 22.9 |
| DDGS | 17 | 3204.5 | 1201.3–6480.6 | 94.1 | 5.9 | 0 | 47.0 | 47.0 |
| Feed | 56 | 755.1 | 214.9–3449.3 | 83.9 | 16.1 | 62.5 | 17.9 | 3.5 |
| Corn | 47 | 445.3 | 200.9–2274.1 | 87.2 | 12.8 | 76.6 | 10.6 | 0 |
| Wheat bran | 15 | 1849.86 | 616.0–3123.4 | 100 | 0 | 26.7 | 66.7 | 6.6 |
| Wheat | 31 | 1338.1 | 204.8–5960.0 | 100 | 0 | 48.4 | 48.4 | 3.2 |
| Corn meal | 27 | 279.2 | 226.3–899.5 | 63.0 | 37 | 63.0 | 0 | 0 |
| Number | Composition | Parameters |
|---|---|---|
| 1 | Preprocessed plates | 96-well transparent microplates (coated with 0.3 ng mL−1 DON-OVA and blocked with 1% OVA) |
| 2 | DON standard | 5000 ng mL−1 in methanol (Dilute to serial standard solution using working buffer before use) |
| 3 | Antibody solution | 6 mL (1.2 ng mL−1 anti-DON McAb in PBS with 2% BSA and 0.05% NaN3) |
| 4 | GAM-HRP solution | 6mL (25 ng mL−1 GAM-HRP in PBS with 2% BSA and 0.05% NaN3) |
| 5 | Color substrate buffer | 15 mL (0.4 mmol L−1 TMB and 3 mmol L−1 H2O2 in citrate buffer, pH 5.0) |
| 6 | Stop buffer | 7 mL (2 mol L−1 H2SO4 in H2O) |
| 7 | 10 × Washing buffer | 40 mL (10 × PBST, pH 7.4) |
| 8 | 1 × Working buffer | 50 mL (5% methanol, 0.2 mol L−1 Na+, pH 7.4 in PBS) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Sun, M.; Hong, X.; Duan, J.; Du, D. Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit. Toxins 2019, 11, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010006
Li M, Sun M, Hong X, Duan J, Du D. Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit. Toxins. 2019; 11(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010006
Chicago/Turabian StyleLi, Ming, Mingna Sun, Xia Hong, Jinsheng Duan, and Daolin Du. 2019. "Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit" Toxins 11, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010006