Clinical Features and Management of Snakebite Envenoming in French Guiana
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Coagulation Disorders
3.2. Systemic Bleeding
3.3. Antivenom
3.4. Adverse Reaction to Antivenom
4. Conclusions
5. Materials and Methods
5.1. Definitions
5.2. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mutricy, R.; Heckmann, X.; Douine, M.; Marty, C.; Jolivet, A.; Lambert, V.; Perotti, F.; Boels, D.; Larréché, S.; Chippaux, J.-P.; et al. High mortality due to snakebites in French Guiana: Time has come to re-evaluate medical management protocols. PLoS Negl. Trop. Dis. 2018, 12, e0006482. [Google Scholar] [CrossRef] [Green Version]
- Kallel, H.; Mayence, C.; Houcke, S.; Mathien, C.; Mehdaoui, H.; Gutiérrez, J.M.; Megarbane, B.; Hommel, D.; Resiere, D. Severe snakebite envenomation in French Guiana: When antivenom is not available. Toxicon 2018, 146, 87–90. [Google Scholar] [CrossRef] [PubMed]
- WHO. Snakebite Envenoming: A Strategy for Prevention and Control; WHO: Geneva, Switzerland, 2019; pp. 837–838. [Google Scholar]
- Kallel, H.; Hommel, D.; Mehdaoui, H.; Megarbane, B.; Resiere, D. Snakebites in French Guiana: Conclusions of an international symposium. Toxicon 2018, 146, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Nadaud, A.; Perotti, F.; de Haro, L.; Boels, D. Snake envenomations in French Guiana: First clinical assessment of an antivenom imported from Mexico. Anaesth. Crit. Care Pain Med. 2019, 38, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Ythier, E. A synopsis of the scorpion fauna of French Guiana, with description of four new species. Zookeys 2018, 764, 27–90. [Google Scholar] [CrossRef] [PubMed]
- Météo France Catalogue sédimentologique des Côtes Françaises—Guyane. 2018. Available online: http:https://meteofrance.com/ (accessed on 10 February 2020).
- le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.A.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Alves, E.C.; Santos, A.S.; Pereira, J.P.T.; Sarraff, L.K.S.; Nascimento, E.F.; Sousa, J.D.D.B.; Sampaio, V.S.; de Lacerda, M.V.G.; Sachett, J.A.G.; et al. Factors associated with systemic bleeding in bothrops envenomation in a tertiary hospital in the Brazilian Amazon. Toxins (Basel) 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Roriz, K.R.P.S.; Zaqueo, K.D.; Setubal, S.S.; Katsuragawa, T.H.; da Silva, R.R.; Fernandes, C.F.C.; Cardoso, L.A.P.; Rodrigues, M.M.D.S.; Soares, A.M.; Stábeli, R.G.; et al. Epidemiological study of snakebite cases in Brazilian Western Amazonia. Rev. Soc. Bras. Med. Trop. 2018, 51, 338–346. [Google Scholar] [CrossRef]
- Alves, E.C.; Sachett, J.A.; Sampaio, V.S.; Sousa, J.D.D.B.; de Oliveira, S.S.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, e0202361. [Google Scholar] [CrossRef] [PubMed]
- Mutricy, R.; Egmann, G.; Marty, C.; Houcke, S.; Adenis, A.; Douine, M.; Nacher, M.; Epelboin, L. Predictors of complications of snake envenomation in Cayenne, French Guiana, 2007–2015. Intensive Care Med. 2018, 44, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Valente-Aguiar, M.S.; Silva, B.G.D.C.E.; Magalhães, T.; Dinis-Oliveira, R.J. Compartment syndrome following bothrops snakebite leads to decompressive fasciotomies. Case Rep. Med. 2019, 2019, 6324569. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; de Capitani, E.M.; Hyslop, S.; Mello, S.M.; Madureira, P.R.; Zanardi, V.; Ferreira, D.M.; Meirelles, G.V.; Fernandes, L.C.R. Compartment syndrome after Bothrops jararaca snakebite: Monitoring, treatment, and outcome. Clin. Toxicol. 2010, 48, 57–60. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Névière, R.; Olive, C.; Severyns, M.; Beaudoin, A.; Florentin, J.; Brouste, Y.; Banydeen, R.; Cabié, A.; et al. Infectious complications following snakebite by bothrops lanceolatus in martinique: A case series. Am. J. Trop. Med. Hyg. 2019, 102, 232–240. [Google Scholar] [CrossRef]
- Résière, D.; Olive, C.; Kallel, H.; Cabié, A.; Névière, R.; Mégarbane, B.; Gutiérrez, J.M.; Mehdaoui, H. Oral microbiota of the snake bothrops lanceolatus in Martinique. Int. J. Environ. Res. Public Health 2018, 15, 1212. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon 2009, 54, 976–987. [Google Scholar] [CrossRef]
- de Brito-Sousa, J.D.; Sachett, J.A.G.; de Oliveira, S.S.; Mendonça-da-Silva, I.; Marques, H.O.; de Lacerda, M.V.G.; Fan, H.W.; Monteiro, W.M. Accuracy of the lee-white clotting time performed in the hospital routine to detect coagulopathy in Bothrops atrox Envenomation. Am. J. Trop. Med. Hyg. 2018, 98, 1547–1551. [Google Scholar] [CrossRef]
- White, J. Snake venoms and coagulopathy. Toxicon 2005, 45, 951–967. [Google Scholar] [CrossRef]
- Bernal, J.C.C.; Bisneto, P.F.; Pereira, J.P.T.; Ibiapina, H.N.D.S.; Sarraff, L.K.S.; Monteiro-Júnior, C.; da Silva Pereira, H.; Santos, B.; de Moura, V.M.; de Oliveira, S.S.; et al. Bad things come in small packages: Predicting venom-induced coagulopathy in Bothrops atrox bites using snake ontogenetic parameters. Clin. Toxicol. 2019, 58, 1–9. [Google Scholar] [CrossRef]
- Sano-Martins, I.S.; Fan, H.W.; Castro, S.C.; Tomy, S.C.; Franca, F.O.; Jorge, M.T.; Kamiguti, A.S.; Warrell, D.A.; Theakston, R.D. Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Butantan Institute Antivenom Study Group. Toxicon 1994, 32, 1045–1050. [Google Scholar] [CrossRef]
- de Oliveira, S.S.; Alves, E.C.; Santos, A.D.S.; Nascimento, E.F.; Pereira, J.P.T.; da Silva, I.M.; Sachett, J.; Ibiapina, H.N.D.S.; Sarraf, L.K.S.; Bernal, J.C.C.; et al. Bothrops snakebites in the Amazon: Recovery from hemostatic disorders after Brazilian antivenom therapy. Clin. Toxicol. 2019, 58, 1–9. [Google Scholar] [CrossRef]
- Heckmann, X.; Lambert, V.; Mion, G.; Ehrhardt, A.; Marty, C.; Perotti, F.; Carod, J.-F.; Jolivet, A.; Boels, D.; Lehida Andi, I.; et al. Failure of a Mexican antivenom on recovery from snakebite-related coagulopathy in French Guiana. Clin. Toxicol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Freitas-de-Sousa, L.A.; Colombini, M.; Lopes-Ferreira, M.; Serrano, S.M.T.; Moura-da-Silva, A.M. Insights into the mechanisms involved in strong hemorrhage and dermonecrosis induced by Atroxlysin-Ia, a PI-class snake venom metalloproteinase. Toxins 2017, 9, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintra, A.C.O.; de Toni, L.G.B.; Sartim, M.A.; Franco, J.J.; Caetano, R.C.; Murakami, M.T.; Sampaio, S.V. Batroxase, a new metalloproteinase from B. Atrox snake venom with strong fibrinolytic activity. Toxicon 2012, 60, 70–82. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Schneider, F.S.; Yarleque, A.; Borges, M.H.; Richardson, M.; Figueiredo, S.G.; Evangelista, K.S.; Eble, J.A. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on blood vessel ECM and platelets. Arch. Biochem. Biophys. 2010, 496, 9–20. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Amazonas, D.R.; Sousa, L.F.; Sant’Anna, S.S.; Nishiyama, M.Y.; Serrano, S.M.T.; Junqueira-de-Azevedo, I.L.M.; Chalkidis, H.M.; Moura-da-Silva, A.M.; Mourão, R.H.V. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of Batroxrhagin, the predominant class PIII metalloproteinase from the venom of this species. Biochimie 2015, 118, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Pardal, P.P.D.O.; Souza, S.M.; Monteiro, M.R.D.C.D.C.; Fan, H.W.; Cardoso, J.L.C.; França, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; de Sousa-E-Silva, M.C.C.; Colombini, M.; et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Fan, H.W.; Vigilato, M.A.N.; Pompei, J.C.A.; Gutiérrez, J.M. Situación de los laboratorios públicos productores de antivenenos en América Latina. Rev. Panam. Salud Publica 2019, 43, e92. [Google Scholar] [CrossRef] [Green Version]
- Segura, A.; Castillo, M.C.; Núñez, V.; Yarlequé, A.; Gonçalves, L.R.C.; Villalta, M.; Bonilla, C.; Herrera, M.; Vargas, M.; Fernández, M.; et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010, 56, 980–989. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Preclinical assessment of the neutralizing efficacy of snake antivenoms in Latin America and the Caribbean: A review. Toxicon 2018, 146, 138–150. [Google Scholar] [CrossRef]
- Smalligan, R.; Cole, J.; Brito, N.; Laing, G.D.; Mertz, B.L.; Manock, S.; Maudlin, J.; Quist, B.; Holland, G.; Nelson, S.; et al. Crotaline snake bite in the ecuadorian amazon: Randomised double blind comparative trial of three South American polyspecific antivenoms. BMJ 2004, 329, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça-da-Silva, I.; Tavares, A.M.; Sachett, J.; Sardinha, J.F.; Zaparolli, L.; Santos, M.F.G.; Lacerda, M.; Monteiro, W.M. Safety and efficacy of a freeze-dried trivalent antivenom for snakebites in the Brazilian Amazon: An open randomized controlled phase IIb clinical trial. PLoS Negl. Trop. Dis. 2017, 11, e0006068. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Villalta, M.; Arias, A.S.; Kallel, H.; Nèviére, R.; Vidal, N.; Mehdaoui, H.; Gutiérrez, J.M. Snakebite envenoming in French Guiana: Assessment of the preclinical efficacy against the venom of Bothrops atrox of two polyspecific antivenoms. Toxicon 2020, 173, 1–4. [Google Scholar] [CrossRef] [PubMed]
- da Siva, A.M.; Monteiro, W.M.; Bernarde, P.S. Popular names for bushmaster (Lachesis muta) and lancehead (Bothrops atrox) snakes in the Alto Juruá region: Repercussions for clinical-epidemiological diagnosis and surveillance. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180140. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; da Siva, A.M.; Amaral, G.L.G.D.; Ortega, G.P.; Monteiro, W.M.; Bernarde, P.S. The deadliest snake according to ethnobiological perception of the population of the Alto Juruá region, western Brazilian Amazonia. Rev. Soc. Bras. Med. Trop. 2019, 53, e20190305. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Patients | Without Antivenom | With Antivenom | p | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Nb | Result | Nb | Result | Nb | Result | |
| Age, years | 133 | 42 (28–52) | 50 | 47 (28–53) | 76 | 40 (29–52) | 0.595 |
| Male gender | 133 | 93 (69.9%) | 50 | 37 (74%) | 76 | 52 (68.4%) | 0.501 |
| BMI, Kg/m2 | 117 | 24 (21–27) | 48 | 24 (22–27) | 62 | 24 (21–27) | 0.866 |
| SAPS | 133 | 13 (6–18) | 50 | 13 (8–19) | 76 | 13 (6–18) | 0.127 |
| History | 133 | 40 (30.1%) | 50 | 14 (28%) | 76 | 24 (31.6%) | 0.668 |
| Hypertension | 133 | 11 (8.3%) | 50 | 2 (4%) | 76 | 8 (10.5%) | 0.185 |
| Alcohol abuse | 133 | 6 (4.5%) | 50 | 4 (8%) | 76 | 2 (2.6%) | 0.166 |
| Duration | |||||||
| Time from SB to Hospitalization | 133 | 7:30 (2:00–11:00) | 50 | 11:55 (5:00–27:30) | 76 | 5:12 (1:08–18:05) | 0.000 |
| Time from Hospitalization to ICU | 128 | 1:39 (0:00–6:22) | 50 | 3:15 (0:00–6:04) | 71 | 1:30 (0:00–3:55) | 0.020 |
| Time from SB to ICU | 128 | 19:00 (5:03–22:03) | 50 | 18:35 (9:33–27:37) | 71 | 9:00 (4:45–18:10) | 0.000 |
| Snake Bite | |||||||
| Grade I envenomation | 133 | 51 (38.3%) | 50 | 14 (28%) | 76 | 32 (42.1%) | 0.108a |
| Grade II envenomation | 133 | 47 (35.3%) | 50 | 18 (36%) | 76 | 28 (36.8%) | 0.381b |
| Grade III envenomation | 133 | 35 (26.2%) | 50 | 18 (36%) | 76 | 16 (21.1%) | 0.064c |
| Clinical features | |||||||
| Snake identification | 133 | 68 (51.1%) | 50 | 24 (48%) | 76 | 39 (51.3%) | 0.716 |
| Oedema | 133 | 131 (98.5%) | 50 | 49 (98%) | 76 | 75 (98.7%) | 0.764 |
| Nb of involved segments | 133 | 2 (1–3) | 50 | 2 (2–3) | 76 | 2 (1–2) | 0.091 |
| Local hemorrhage | 133 | 19 (14.3%) | 50 | 5 (10%) | 76 | 14 (18.4%) | 0.196 |
| Necrosis | 133 | 14 (10.5%) | 50 | 5 (10%) | 76 | 9 (11.8%) | 0.748 |
| Blister | 133 | 19 (14.3%) | 50 | 15 (30%) | 76 | 4 (5.3%) | 0.000 |
| Pain | 133 | 130 (97.7%) | 50 | 47 (94%) | 76 | 76 (100%) | 0.031 |
| Mean arterial pressure, mmHg | 133 | 95 (88–102) | 50 | 93 (86–103) | 76 | 95 (89–102) | 0.578 |
| Cardiac rythm, beat/min | 133 | 78 (67–91) | 50 | 84 (72–94) | 76 | 76 (66–90) | 0.213 |
| Temperature (°C) | 133 | 37 (37–37) | 50 | 37 (37–38) | 76 | 37 (37–37) | 0.001 |
| SpO2 (%) | 133 | 99 (98–100) | 50 | 99 (97–100) | 76 | 100 (98–100) | 0.274 |
| Shock | 133 | 2 (1.5%) | 50 | 2 (4%) | 76 | 0 (0%) | 0.079 |
| Renal failure | 133 | 20 (15%) | 50 | 10 (20%) | 76 | 10 (13.2%) | 0.304 |
| Systemic hemorrhage | 133 | 24 (18%) | 50 | 12 (24%) | 76 | 11 (14.5%) | 0.176 |
| All Patients | Without Antivenom | With Antivenom | p | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Nb | Result | Nb | Result | Nb | Result | |
| Dialysis | 20 | 10 (50%) | 10 | 6 (60%) | 10 | 4 (40%) | 0.371 |
| Cathecholamines | 133 | 2 (1.5%) | 50 | 2 (4%) | 76 | 0 (0%) | 0.079 |
| Mechanical Ventilation (MV) | 133 | 3 (2.3%) | 50 | 3 (6%) | 76 | 0 (0%) | 0.031 |
| Duration of MV, days | 3 | 16 (9–28) | 3 | 16 (9–28) | 0 | ||
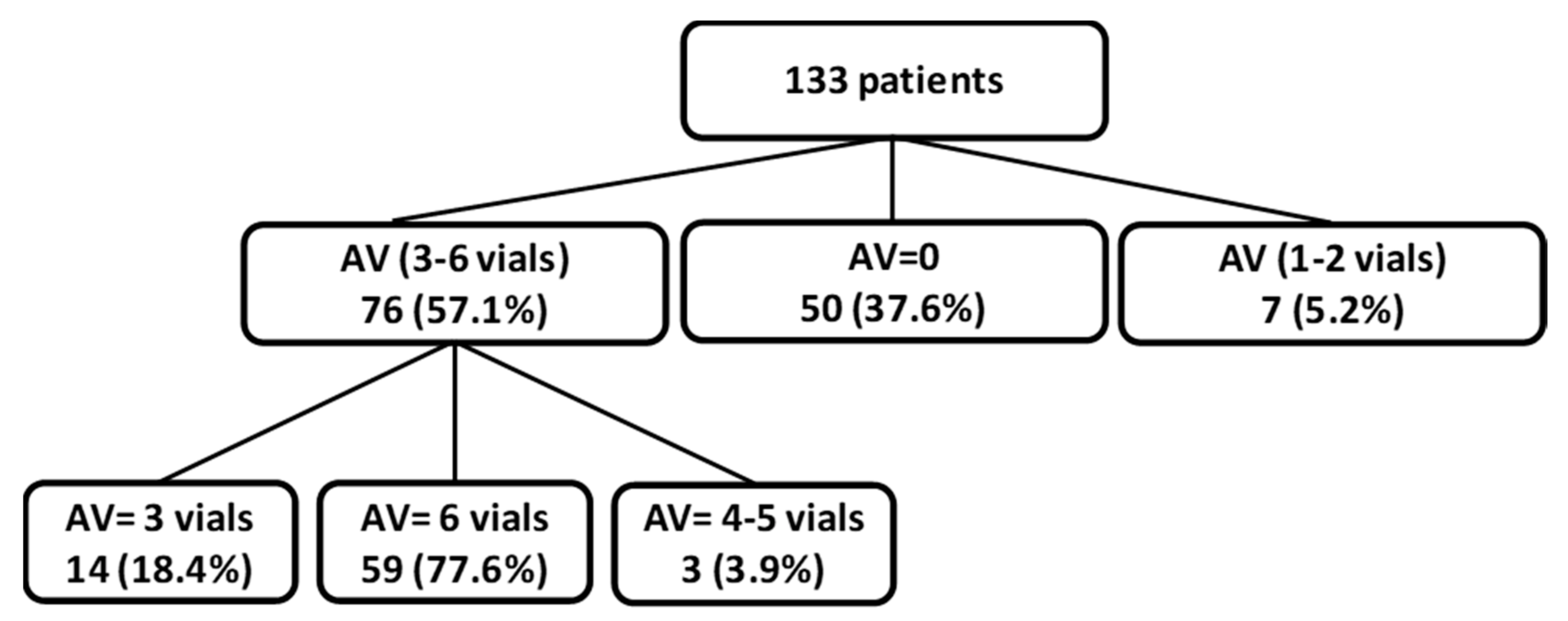
| Antivenom | 133 | 83 (62.4%) | 50 | 0 (0%) | 76 | 76 (100%) | - |
| Time from SB to AV, hour | 83 | 9:00 (5:22–20:40) | 0 | - | 76 | 9:00 (5:26–21:00) | - |
| AV 3 to 6 vials | 83 | 76 (91.6%) | 0 | - | 76 | 76 (100%) | - |
| Prescribed dose | 83 | 6 (6–6) | 0 | - | 76 | 6 (6–6) | - |
| Received dose | 83 | 6 (4–6) | 0 | - | 76 | 6 (6–6) | - |
| Early adverse reaction | 83 | 17 (20.5%) | 0 | - | 76 | 10 (13.2%) | - |
| Surgery | 133 | 35 (26.3%) | 50 | 15 (30%) | 76 | 19 (25%) | 0.536 |
| Time from SB to surgery, days | 35 | 7 (6–9) | 15 | 8 (6 - 10) | 19 | 7 (5–9) | 0.137 |
| Necrosectomy | 35 | 16 (46%) | 15 | 7 (47%) | 19 | 9 (47%) | 0.968 |
| Infection | 133 | 43 (32.3%) | 50 | 19 (38%) | 76 | 23 (30.3%) | 0.367 |
| Abscess | 43 | 28 (65.1%) | 19 | 11 (57.9%) | 23 | 16 (69.6%) | 0.432 |
| Necrotizing fasciitis | 43 | 8 (18.6%) | 19 | 3 (15.8%) | 23 | 5 (21.7%) | 0.625 |
| Cellulitis | 43 | 12 (27.9%) | 19 | 6 (31.6%) | 23 | 6 (26.1%) | 0.695 |
| Time from SB to infection, days | 42 | 6 (3–8) | 19 | 5 (3–9) | 22 | 6 (3–7) | 0.344 |
| Outcome | |||||||
| ICU Length of Stay, days | 128 | 3 (3–5) | 50 | 4 (3–7) | 71 | 3 (3–4) | 0.009 |
| Hospital LOS, days | 133 | 10 (6–13) | 50 | 11 (7–20) | 76 | 9 (6–13) | 0.002 |
| Survival | 133 | 131 (98.5%) | 50 | 48 (96%) | 76 | 76 (100%) | 0.079 |
| All Patients | Without Antivenom | With Antivenom | p | ||||
| Parameter | Nbval | Result | Nb | Result | Nb | Result | |
| Hemolysis | 133 | 37 (27.8%) | 50 | 14 (28%) | 76 | 21 (27.6%) | 0.964 |
| Time from SB to end of hemolysis | 128 | 18:28 (6:42–30:40) | 47 | 22:00 (15:12–74:35) | 74 | 16:30 (3:30–24:52) | 0.002 |
| Rhabdomyolysis | 133 | 49 (36.8%) | 50 | 15 (30%) | 76 | 33 (43.4%) | 0.129 |
| Time from SB to normal CK | 116 | 18:15 (7:21–28:07) | 45 | 22:00 (13:00–63:30) | 64 | 14:55 (5:25–22:32) | 0.136 |
| Hyperlactacidemia | 132 | 27 (20.5%) | 50 | 10 (20%) | 75 | 14 (18.7%) | 0.853 |
| Coagulation | |||||||
| Defibrinogenation | 133 | 124 (93.2%) | 50 | 42 (84%) | 76 | 75 (98.7%) | 0.002 |
| Time from SB to normal fibrinogen | 130 | 29:00 (22:18–45:55) | 47 | 47:00 (28:30–96:13) | 76 | 25:30 (20:42–32:45) | 0.000 |
| International Normalized Ratio (INR) | 133 | 104 (78.2%) | 50 | 32 (64%) | 76 | 66 (86.8%) | 0.003 |
| Time from SB to normal INR | 132 | 28:21 (17:55–54:54) | 49 | 58:15 (27:30–91:26) | 76 | 22:32 (17:00–35:03) | 0.000 |
| Partial thromboplastin time (PTT) | 133 | 74 (55.6%) | 50 | 23 (46%) | 76 | 47 (61.8%) | 0.080 |
| Time from SB to normal PTT | 132 | 18:20 (12:55–27:30) | 49 | 25:00 (16:30–58:40) | 76 | 16:05 (11:00–21:15) | 0.000 |
| Thrombocytopenia | 133 | 52 (39.1%) | 50 | 22 (44%) | 76 | 29 (38.2%) | 0.513 |
| Time from SB to normal Platelet count | 128 | 19:49 (5:37–107:23) | 48 | 40:37 (12:47–156:34) | 73 | 18:00 (3:03–62:00) | 0.010 |
| Platelet count, Giga/L | 52 | 116 (75–130) | 22 | 95 (73–121) | 29 | 121 (99–132) | 0.145 |
| Decreased Factor II | 133 | 48 (36.1%) | 50 | 13 (26%) | 76 | 34 (44.7%) | 0.033 |
| Time from SB to normal Factor II | 110 | 17:29 (6:49–34:02) | 34 | 24:55 (15:24–66:35) | 69 | 15:10 (5:30–30:30) | 0.000 |
| Decreased Factor V | 133 | 66 (49.6%) | 50 | 13 (26%) | 76 | 49 (64.5%) | 0.000 |
| Time from SB to normal Factor V | 108 | 23:30 (15:05–37:34) | 38 | 31:42 (19:00–65:52) | 64 | 19:42 (11:00–30:30) | 0.000 |
| Decreased Factor VII | 133 | 45 (33.8%) | 50 | 11 (22%) | 76 | 31 (40.8%) | 0.029 |
| Time from SB to normal Factor VII | 95 | 15:10 (5:05–38:05) | 33 | 22:25 (12:11–78:10) | 56 | 8:00 (3:23–20:02) | 0.000 |
| Decreased Factor VIII | 133 | 14 (10.5%) | 50 | 4 (8%) | 76 | 9 (11.8%) | 0.488 |
| Time from SB to normal Factor VIII | 120 | 13:22 (6:07–22:52) | 38 | 21:30 (14:00–57:56) | 75 | 10:20 (5:05–18:48) | 0.000 |
| Decreased Factor IX | 133 | 4 (3%) | 50 | 0 (0%) | 76 | 4 (5.3%) | 0.099 |
| Time from SB to normal Factor IX | 123 | 12:20 (5:22–22:21) | 40 | 20:35 (10:52–40:28) | 76 | 9:50 (3:51–18:42) | 0,000 |
| Decreased Factor X | 133 | 19 (14.3%) | 50 | 8 (16%) | 76 | 11 (14.5%) | 0.815 |
| Time from SB to normal Factor X | 114 | 14:05 (5:32–25:27) | 35 | 22:00 (12:35–81:20) | 72 | 10:27 (3:51–20:32) | 0.000 |
| Decreased Factor XI | 133 | 7 (5.3%) | 50 | 0 (0%) | 76 | 6 (7.9%) | 0.042 |
| Time from SB to normal Factor XI | 122 | 12:42 (5:56–22:57) | 40 | 20:35 (10:52–40:28) | 75 | 10:00 (3:51–19:15) | 0.000 |
| Decreased Factor XII | 133 | 13 (9.8%) | 50 | 3 (6%) | 76 | 9 (11.8%) | 0.274 |
| Time from SB to normal Factor XII | 113 | 12:50 (5:40–23:00) | 39 | 21:00 (11:35–39:37) | 68 | 10:00 (3:41–19:07) | 0.000 |
| With Antivenom | 3 Vials of Antivenom | 6 Vials of Antivenom | p | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Nb | Result | Nb | Result | Nb | Result | |
| Time from SB to end of hemolysis | 74 | 16:30 (3:30–24:52) | 14 | 20:00 (11:45–29:35) | 57 | 11:00 (2:46–23:30) | 0.048 |
| Rhabdomyolysis | 76 | 33 (43.4%) | 14 | 6 (42.9%) | 59 | 27 (46%) | 0.844 |
| Time from SB to normal CPK | 64 | 14:55 (5:25–22:32) | 13 | 18:50 (11:00–41:02) | 48 | 13:20 (5:10–20:02) | 0.021 |
| Hyperlactacidemia | 75 | 14 (18.7%) | 14 | 1 (7.1%) | 58 | 13 (22%) | 0.195 |
| Coagulation | |||||||
| Defibrinogenation | 76 | 75 (98.7%) | 14 | 14 (100%) | 59 | 58 (98%) | 0.624 |
| Time from SB to normal fibrinogen | 76 | 25:30 (20:42–32:45) | 14 | 33:30 (25:10–44:30) | 59 | 25:10 (20:33–31:00) | 0.064 |
| International Normalized Ratio | 76 | 66 (86.8%) | 14 | 12 (85.7%) | 59 | 52 (88%) | 0.804 |
| Time from SB to normal INR | 76 | 22:32 (17:00–35:03) | 14 | 31:15 (24:22–43:37) | 59 | 20:50 (17:00–33:50) | 0.227 |
| Partial thromboplastin time (PTT) | 76 | 47 (61.8%) | 14 | 9 (64.3%) | 59 | 36 (61%) | 0.821 |
| Time from SB to normal PTT | 76 | 16:05 (11:00–21:15) | 14 | 18:05 (16:02–28:22) | 59 | 16:00 (11:00–20:15) | 0.187 |
| Thrombocytopenia | 76 | 29 (38.2%) | 14 | 7 (50%) | 59 | 22 (37%) | 0.382 |
| Time from SB to normal Platelet count | 73 | 18:00 (3:03–62:00) | 13 | 22:00 (11:00–62:00) | 57 | 18:00 (2:46–64:00) | 0.904 |
| Platelet count, Giga/L | 29 | 121 (99–132) | 7 | 129 (67–133) | 22 | 121 (101–131) | 0.570 |
| Decreased Factor II | 76 | 34 (44.7%) | 14 | 8 (57.1%) | 59 | 25 (42%) | 0.318 |
| Time from SB to normal Factor II | 69 | 15:10 (5:30–30:30) | 11 | 18:00 (13:35–32:30) | 56 | 13:52 (5:07–27:55) | 0.492 |
| Decreased Factor V | 76 | 49 (64.5%) | 14 | 9 (64.3%) | 59 | 37 (63%) | 0.913 |
| Time from SB to normal Factor V | 64 | 19:42 (11:00–30:30) | 12 | 25:40 (16:07–29:37) | 50 | 17:50 (10:13–30:20) | 0.306 |
| Decreased Factor VII | 76 | 31 (40.8%) | 14 | 5 (35.7%) | 59 | 25 (42%) | 0.649 |
| Time from SB to normal Factor VII | 56 | 8:00 (3:23–20:02) | 11 | 18:00 (10:40–24:15) | 43 | 5:40 (2:23–19:25) | 0.451 |
| Decreased Factor VIII | 76 | 9 (11.8%) | 14 | 3 (21.4%) | 59 | 5 (8%) | 0.163 |
| Time from SB to normal Factor VIII | 75 | 10:20 (5:05–18:48) | 14 | 18:00 (11:00–25:15) | 59 | 9:00 (3:51–18:10) | 0.008 |
| Decreased Factor IX | 76 | 4 (5.3%) | 14 | 0 (0%) | 59 | 4 (7%) | 0.316 |
| Time from SB to normal Factor IX | 76 | 9:50 (3:51–18:42) | 14 | 18:00 (9:30–25:15) | 59 | 9:00 (3:40–18:10) | 0.024 |
| Decreased Factor X | 76 | 11 (14.5%) | 14 | 2 (14.3%) | 59 | 9 (15%) | 0.927 |
| Time from SB to normal Factor X | 72 | 10:27 (3:51–20:32) | 14 | 18:00 (11:20–25:15) | 55 | 7:25 (3:16–19:40) | 0.074 |
| Decreased Factor XI | 76 | 6 (7.9%) | 14 | 1 (7.1%) | 59 | 4 (7%) | 0.961 |
| Time from SB to normal Factor XI | 75 | 10:00 (3:51–19:15) | 14 | 18:00 (11:00–25:15) | 59 | 9:25 (3:01–18:28) | 0.020 |
| Decreased Factor XII | 76 | 9 (11.8%) | 14 | 3 (21.4%) | 59 | 6 (10%) | 0.249 |
| Time from SB to normal Factor XII | 68 | 10:00 (3:41–19:07) | 12 | 19:15 (10:30–25:20) | 53 | 7:00 (3:00–18:20) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resiere, D.; Houcke, S.; Pujo, J.M.; Mayence, C.; Mathien, C.; NkontCho, F.; Blaise, N.; Demar, M.P.; Hommel, D.; Kallel, H. Clinical Features and Management of Snakebite Envenoming in French Guiana. Toxins 2020, 12, 662. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100662
Resiere D, Houcke S, Pujo JM, Mayence C, Mathien C, NkontCho F, Blaise N, Demar MP, Hommel D, Kallel H. Clinical Features and Management of Snakebite Envenoming in French Guiana. Toxins. 2020; 12(10):662. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100662
Chicago/Turabian StyleResiere, Dabor, Stéphanie Houcke, Jean Marc Pujo, Claire Mayence, Cyrille Mathien, Flaubert NkontCho, Nicaise Blaise, Magalie Pierre Demar, Didier Hommel, and Hatem Kallel. 2020. "Clinical Features and Management of Snakebite Envenoming in French Guiana" Toxins 12, no. 10: 662. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100662





