Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease
Abstract
:1. Introduction
2. Results
2.1. Working Model of CKD Mouse with AAN and Identification of Renal Function and Uremic Cachexia
2.2. AAN Mice Exhibit the Most Prominent Renal Atropy, TIF and Necrosis of Tubular Cells, Whereas PE Treatment Ameliorated Such Renal Damages
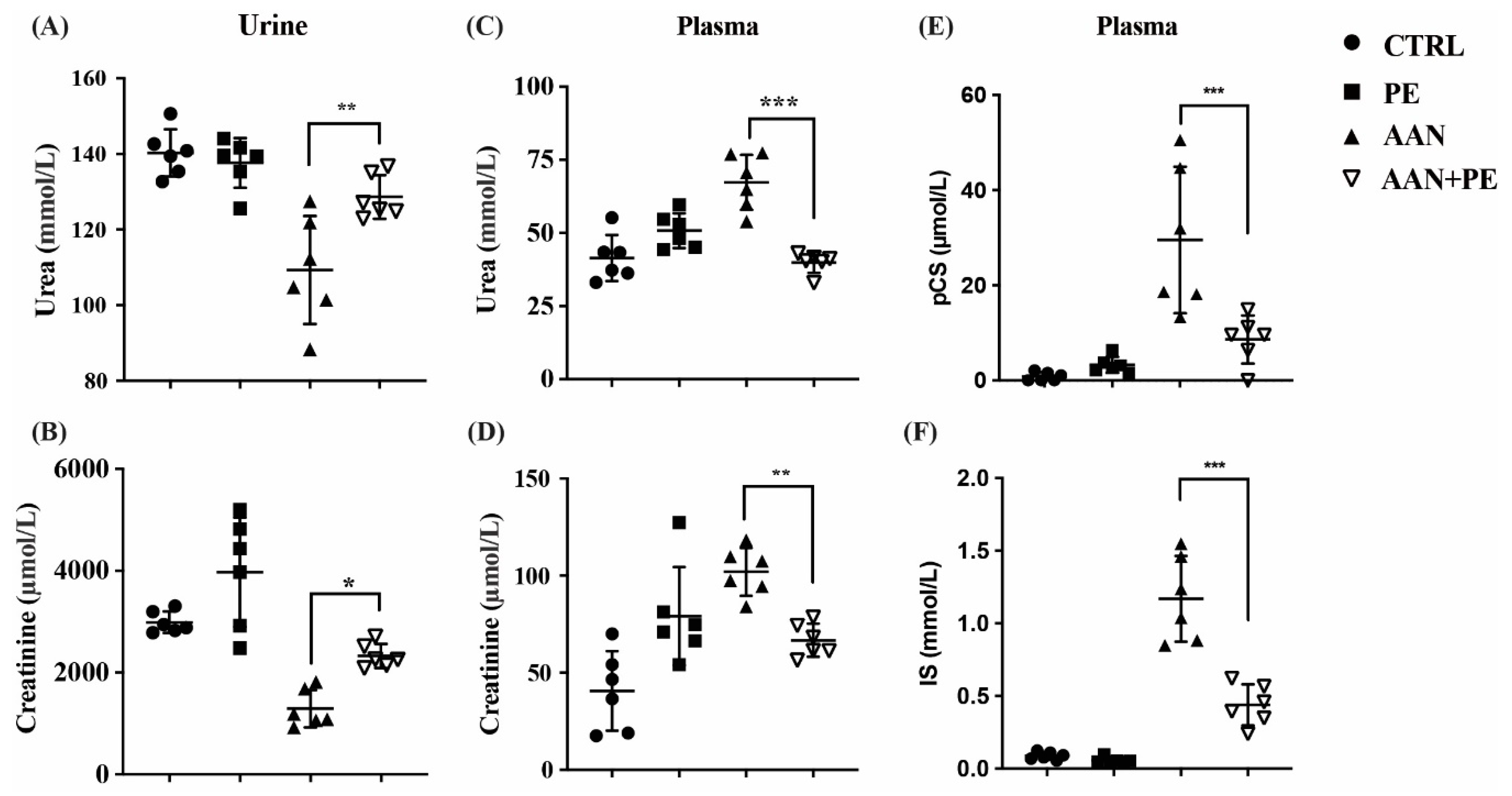
2.3. PE Treatment Imporved Renal Function Indicators and Plasma Retention of Uremic Toxins (IS and pCS) in AAN Model
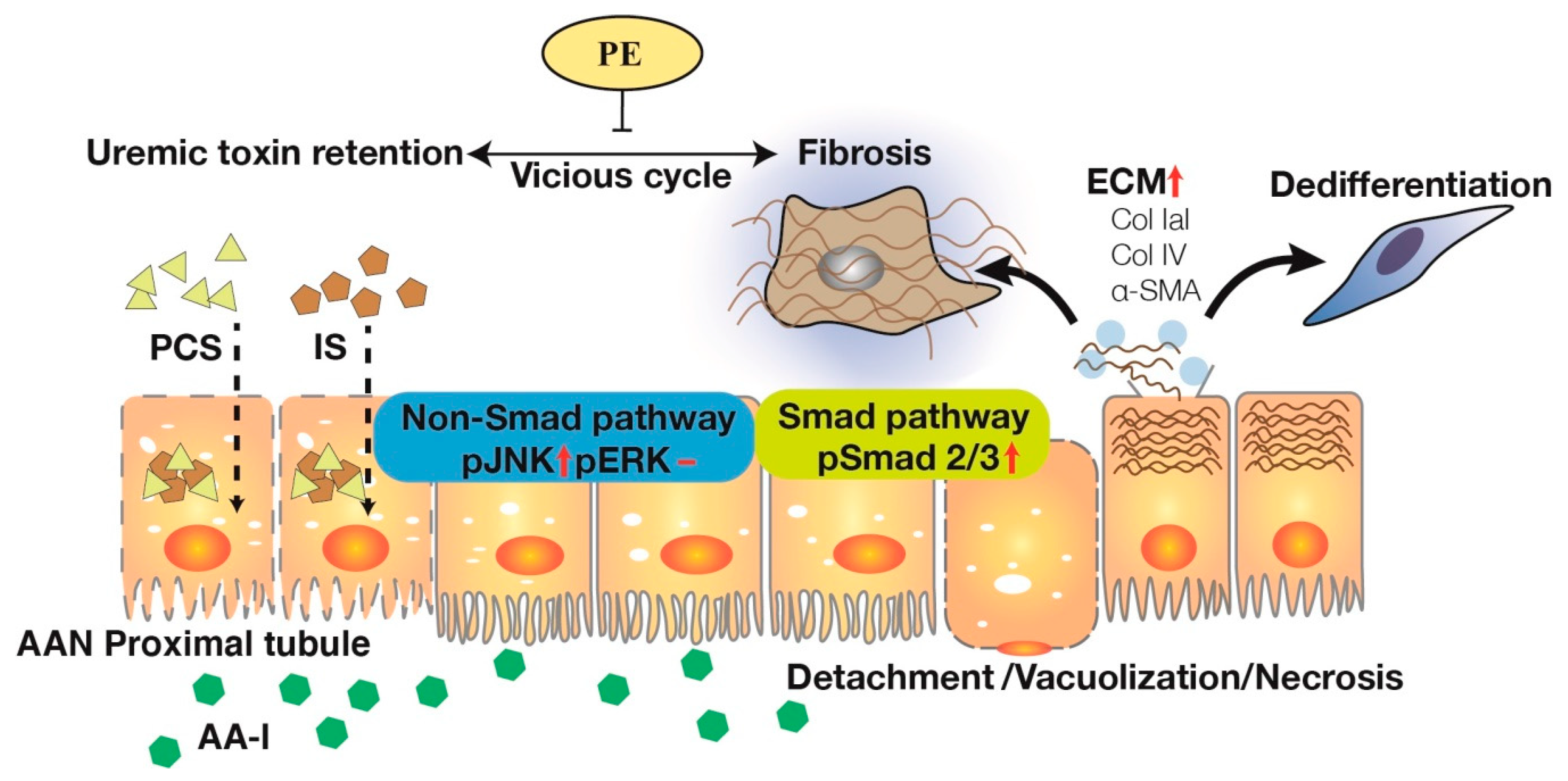
2.4. PE Treatment Attenuated Tissue Expressions of TIF, Fibrotic EMT and TGF-β Signaling Transduction Pathways
3. Discussion
4. Materials and Methods
4.1. Creating Animal Models to Mimic TIF in Humans with Progressive CKD
4.2. Tissue Preparation for Histopathological Evaluation of H&E Stain
4.3. Masson’s Trichrome Staining Method
4.4. Biochemical Assays of Urea nitrogen, Creatinine (Cr), PCS and IS
4.5. Western Blot Analysis
4.6. Statistical Analysis of Data
Author Contributions
Funding
Conflicts of Interest
References
- Jadot, I.; Declèves, A.-E.; Nortier, J.; Caron, N. An integrated view of aristolochic acid nephropathy: Update of the literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional management of chronic kidney disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and clinical impact of organic uremic retention solutes: A comprehensive update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, H.A.; Wilmer, M.J.; Reijnders, D.; Jansen, J.; van den Broek, P.H.; Forkink, M.; Schepers, E.; Glorieux, G.; Vanholder, R.; van den Heuvel, L.P.; et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim. Biophys. Acta 2013, 1832, 142–150. [Google Scholar] [CrossRef]
- Satoh, M.; Hayashi, H.; Watanabe, M.; Ueda, K.; Yamato, H.; Yoshioka, T.; Motojima, M. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp. Nephrol. 2003, 95, e111–e118. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, H.C.; Fogo, A.B. A perspective on chronic kidney disease progression. Am. J. Physiol. Ren. Physiol. 2017, 312, F375–f384. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-F.; Liang, S.-S.; Thanasekaran, P.; Chang, H.-W.; Wen, L.-L.; Chen, C.-H.; Liou, J.-C.; Yeh, J.-C.; Liu, S.-H.; Dai, H.-M.; et al. Translational medicine in pulmonary-renal crosstalk: Therapeutic targeting of p-cresyl sulfate triggered nonspecific ros and chemoattractants in dyspneic patients with uremic lung injury. J. Clin. Med. 2018, 7, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutsaers, H.A.; Stribos, E.G.; Glorieux, G.; Vanholder, R.; Olinga, P. Chronic kidney disease and fibrosis: The role of uremic retention solutes. Front. Med. 2015, 2, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Zhang, Y.E. Smad-dependent and smad-independent pathways in tgf-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Potenta, S.; Kalluri, R. Transforming growth factor-β2 promotes snail-mediated endothelial-mesenchymal transition through convergence of smad-dependent and smad-independent signalling. Biochem. J. 2011, 437, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Nai, Y.S.; Chen, Y.W.; Hua, K.F. Mechanistic insight into the attenuation of gouty inflammation by taiwanese green propolis via inhibition of the nlrp3 inflammasome. J. Cell Physiol. 2019, 234, 4081–4094. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chien, Y.W.; Chang, M.L.; Hou, C.C.; Chan, C.H.; Tang, H.W.; Huang, H.Y. Taiwanese green propolis ethanol extract delays the progression of type 2 diabetes mellitus in rats treated with streptozotocin/high-fat diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins from taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.N.; Wu, C.L.; Lin, J.K. Apoptosis of human melanoma cells induced by the novel compounds propolin a and propolin b from taiwenese propolis. Cancer Lett. 2007, 245, 218–231. [Google Scholar] [CrossRef]
- Chang, J.-F.; Yeh, J.-C.; Ho, C.-T.; Liu, S.-H.; Hsieh, C.-Y.; Wang, T.-M.; Chang, S.-W.; Lee, I.T.; Huang, K.-Y.; Wang, J.-Y.; et al. Targeting ros and cpla2/cox2 expressions ameliorated renal damage in obese mice with endotoxemia. Int. J. Mol. Sci. 2019, 20, 4393. [Google Scholar] [CrossRef] [Green Version]
- Mak, R.H.; Cheung, W.; Cone, R.D.; Marks, D.L. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int. 2006, 69, 794–797. [Google Scholar] [CrossRef] [Green Version]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep. 2016, 6, 36618. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68–70. [Google Scholar] [CrossRef]
- Peres, L.C.; Sethuraman, C.; Al-Adnani, M.; Cohen, M.C. Necrotic epithelial cells in proximal renal tubules of 2nd trimester fetuses: Is this “acute tubular necrosis”? Int. J. Clin. Exp. Pathol. 2012, 5, 326–330. [Google Scholar]
- Huang, L.; Scarpellini, A.; Funck, M.; Verderio, E.A.M.; Johnson, T.S. Development of a chronic kidney disease model in c57bl/6 mice with relevance to human pathology. Nephron Extra 2013, 3, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012, 40, 14s–86s. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Iredale, J.P. Regression of liver fibrosis. Semin. Liver. Dis. 2017, 37, 1–10. [Google Scholar] [PubMed]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Su, T.; Li, X.-M.; Wang, X.; Cai, S.-Q.; Meng, L.-Q.; Zou, W.-Z.; Wang, H.-Y. Aristolochic acid nephropathy: Variation in presentation and prognosis. Nephrol Dial. Transplant. 2012, 27, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic aspects of a newly described nephropathy related to the prolonged use of chinese herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, S.; Higashiyama, Y.; Nishijima, F.; Niwa, T. Creb, nf-kappab, and nadph oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2013, 304, C685–C692. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Bolati, D.; Higashiyama, Y.; Nishijima, F.; Shimizu, K.; Niwa, T. Indoxyl sulfate upregulates renal expression of mcp-1 via production of ros and activation of nf-κb, p53, erk, and jnk in proximal tubular cells. Life Sci. 2012, 90, 525–530. [Google Scholar] [CrossRef]
- Shimizu, H.; Bolati, D.; Adijiang, A.; Muteliefu, G.; Enomoto, A.; Nishijima, F.; Dateki, M.; Niwa, T. Nf-kappab plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2011, 301, C1201–C1212. [Google Scholar] [CrossRef]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of indoxyl sulfate and p-cresol sulfate on chronic kidney disease and mitigating effects of ast-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozdzik, A.A.; Salmon, I.J.; Debelle, F.D.; Decaestecker, C.; Van den Branden, C.; Verbeelen, D.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008, 73, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Wang, D.; Wang, Y.; Li, Y.; Wu, G. Tgf-beta 1/smads signaling stimulates renal interstitial fibrosis in experimental aan. J. Recept. Signal. Transduct. Res. 2009, 29, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Shen, H.; Lu, Y.; Li, H.; Ren, X.; Wu, G. Tgf-beta1/smad7 signaling stimulates renal tubulointerstitial fibrosis induced by aai. J. Recept. Signal. Transduct. Res. 2008, 28, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Lu, D.W.; Zhao, H.; Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Zhao, Y.Y. Central role of dysregulation of tgf-β/smad in ckd progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. Tgf-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into tgf-β/smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Chung, A.C.; Lai, K.N.; Lan, H.Y. Mechanism of chronic aristolochic acid nephropathy: Role of smad3. Am. J. Physiol. Ren. Physiol. 2010, 298, F1006–F1017. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Tgf-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Macias, M.J.; Martin-Malpartida, P.; Massagué, J. Structural determinants of smad function in tgf-β signaling. Trends Biochem. Sci. 2015, 40, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.E. Non-smad pathways in tgf-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-F.; Hsieh, C.-Y.; Lu, K.-C.; Chen, Y.-W.; Liang, S.-S.; Lin, C.-C.; Hung, C.-F.; Liou, J.-C.; Wu, M.-S. Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease. Toxins 2020, 12, 364. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12060364
Chang J-F, Hsieh C-Y, Lu K-C, Chen Y-W, Liang S-S, Lin C-C, Hung C-F, Liou J-C, Wu M-S. Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease. Toxins. 2020; 12(6):364. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12060364
Chicago/Turabian StyleChang, Jia-Feng, Chih-Yu Hsieh, Kuo-Cheng Lu, Yue-Wen Chen, Shih-Shin Liang, Chih-Cheng Lin, Chi-Feng Hung, Jian-Chiun Liou, and Mai-Szu Wu. 2020. "Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease" Toxins 12, no. 6: 364. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12060364




