Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of Pharmacopuncture-EHD (P-EHD)
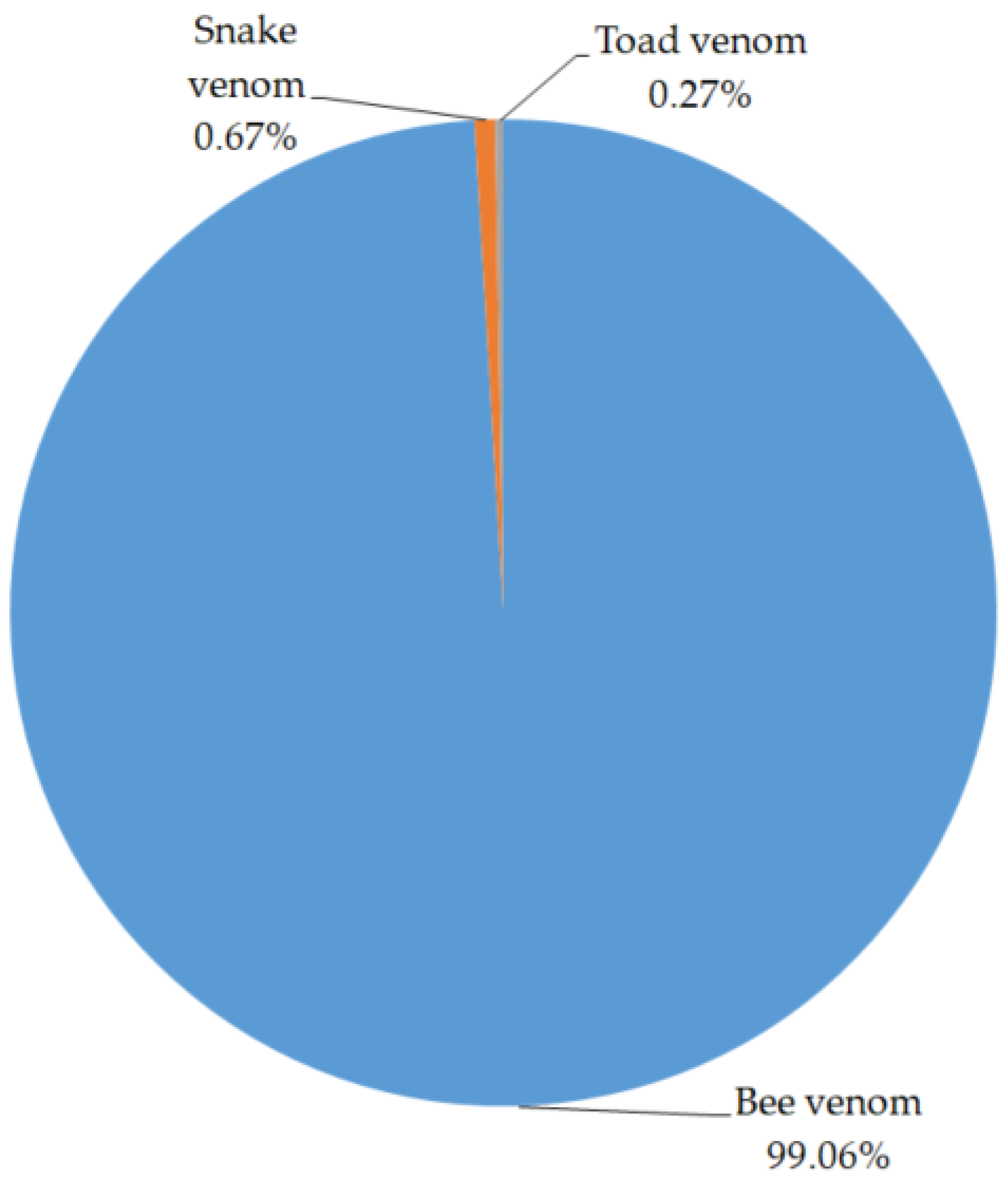
2.2. Preparation Status of Pharmacopuncture
2.3. The Preparation Process of Animal Venom Pharmacopuncture
2.4. Clinical Effectiveness of Animal Venom Therapy
3. Discussion
4. Conclusions
5. Methods
5.1. Overview of the Study
5.2. Study Sample
5.3. Questionnaire Development and Distribution
5.4. Questionnaire Items
5.5. Data Collection
5.6. Data Analysis
5.7. Literature Search of the Production Process and Clinical Effectiveness of Animal Venom Medications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utkin, Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015, 6, 28–33. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Chapter 49—Zootoxins—Reproductive and Developmental Toxicology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Hakim, M.A.; Yang, S.; Lai, R. Centipede Venoms and Their Components: Resources for Potential Therapeutic Applications. Toxins 2015, 7, 4832–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal protein toxins: Origins and therapeutic applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.B.; Kim, K.H. Clinical effectiveness and adverse events of bee venom therapy: A systematic review of randomized controlled trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef]
- El-Aziz, M.A.T.; Soares, A.G.; Stockand, J.D. Snake venoms in drug discovery: Valuable therapeutic tools for life saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, J.M.; Goncalves, B.D.C.; Gomez, M.V.; Vieira, L.B.; Ribeiro, F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.M.; Kim, K.H.; Oh, Y.T.; Kim, J.W.; Yook, T.H. The Analysis of the Recent Research Trend of Pharmacopuncture. J. Soc. Prev. Korean Med. 2018, 22, 55–63. [Google Scholar]
- Park, J.; Lee, H.; Shin, B.C.; Lee, M.S.; Kim, B.; Kim, J.I. Pharmacopuncture in Korea: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2016, 2016, 4683121. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.H.; Shin, B.C.; Park, M.J.; Kim, K.H.; Kim, J.W.; Ryu, J.Y.; Park, J.K. Current status of management on pharmacopuncture in Korea through introduction of an accreditation system. J. Pharmacopunct. 2019, 22, 75–82. [Google Scholar]
- Kim, D.H.; Cho, S.J.; Ko, J.A. Policy Improvement Plan Based on Korean Medicine Use; Health Insurance Review & Assessment Service: Wonju, Korea, 2015. [Google Scholar]
- Ministry of Health and Welfare. Study on Methods to Prove Reasonable Safety and Efficacy of Herbal Medicines; Ministry of Health and Welfare: Sejong, Korea, 2017.
- Ministry of Health and Welfare, National Development Institute of Korean Medicine, Gallup Korea. 2017 Years National Survey for Usage and Consumption of Traditional Herbal Medicine; National Development Institute of Korean Medicine: Seoul, Korea, 2018.
- Kim, J.H.; Kim, Y.K. A study on the facility standard of herbal dispensaries. J. Korean Med. 2017, 38, 81–92. [Google Scholar] [CrossRef]
- Korean Pharmacopuncture Institute. Pharmacopuncturology; Elsevier Korea: Seoul, Korea, 2011. [Google Scholar]
- Hayes, W.K.; Fox, G.A.; Nelsen, D.R. Venom collection from spiders and snakes: Voluntary and involuntary extractions (“Milking”) and venom gland extractions. Methods Mol. Biol. 2020, 2068, 53–71. [Google Scholar] [PubMed]
- Lee, M.S.; Pittler, M.H.; Shin, B.C.; Kong, J.C.; Ernst, E. Bee venom acupuncture for musculoskeletal pain: A review. J. Pain 2008, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Lee, S.H. Effectiveness of bee venom acupuncture in alleviating post-stroke shoulder pain: A systematic review and meta-analysis. J. Integr. Med. 2015, 13, 241–247. [Google Scholar] [CrossRef]
- Shen, L.; Lee, J.H.; Joo, J.C.; Park, S.J.; Song, Y.S. Bee venom acupuncture for shoulder pain: A systematic review and meta-analysis of randomized controlled trials. J. Pharmacopunct. 2020, 23, 44–53. [Google Scholar]
- Koh, P.S.; Seo, B.K.; Cho, N.S.; Park, H.S.; Park, D.S.; Baek, Y.H. Clinical effectiveness of bee venom acupuncture and physiotherapy in the treatment of adhesive capsulitis: A randomized controlled trial. J. Shoulder Elb. Surg. 2013, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Koh, P.S.; Seo, B.K.; Lee, J.W.; Cho, N.S.; Park, H.S.; Park, D.S.; Baek, Y.H. Long-term effectiveness of bee venom acupuncture and physiotherapy in the treatment of adhesive capsulitis: A one-year follow-up analysis of a previous randomized controlled trial. J. Altern. Complement. Med. 2014, 20, 919–924. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.Y.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Park, S.U. Bee venom acupuncture point injection for central post stroke pain: A preliminary single-blind randomized controlled trial. Complement. Ther. Med. 2013, 21, 155–157. [Google Scholar] [CrossRef]
- Seo, B.K.; Han, K.; Kwon, O.; Jo, D.J.; Lee, J.H. Efficacy of bee venom acupuncture for chronic low back pain: A randomized, double-blinded, sham-controlled trial. Toxins 2017, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Shin, B.C.; Kong, J.C.; Park, T.Y.; Yang, C.Y.; Kang, K.W.; Choi, S.M. Bee venom acupuncture for chronic low back pain: A randomised, sham-controlled, triple-blind clinical trial. Eur. J. Integr. Med. 2012, 4, e271–e280. [Google Scholar] [CrossRef]
- Kim, K.T.; Song, H.S. The effectiveness of bee venom acupuncture therapy on the treatment of sprain of L-spine (A randomized controlled trial: Fouble blinding). J. Korean Acupunct. Moxibustion Soc. 2005, 22, 113–120. [Google Scholar]
- Kim, S.K.; Kim, M.C. The affect on delayed onset muscle soreness recovery for ultrasound with bee venom. J. Phys. Ther. Sci 2014, 26, 1419–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.S.; Lee, J.S.; Chung, S.H.; Kim, S.S. The effect of bee venom acupuncture on patient with hernation of nucleus pulposus of lumbar spine. J. Orient. Rehab. Med. 2003, 13, 87–101. [Google Scholar]
- Yu, S.M.; Lee, J.Y.; Kwon, K.R.; Lee, H.S. Comparative study on acupuncture, bee venom acupuncture, and bee venom pharmacopuncture on the treatment of herniation of nucleus pulpous. J. Korean Acupunct. Moxibustion Soc. 2006, 23, 39–54. [Google Scholar]
- Kwon, Y.B.; Kim, J.H.; Yoon, J.H.; Lee, J.D.; Han, H.J.; Mar, W.C.; Beitz, A.J.; Lee, J.H. The analgesic efficacy of bee venom acupuncture for knee osteoarthritis: A comparative study with needle acupuncture. Am. J. Chin. Med. 2001, 29, 187–199. [Google Scholar] [CrossRef]
- Conrad, V.J.; Hazan, L.L.; Latorre, A.J.; Jakubowska, A.; Kim, C.M.H. Efficacy and safety of honey bee venom (Apis mellifera) dermal injections to treat osteoarthritis knee pain and physical disability: A randomized controlled trial. J. Altern. Complement. Med. 2019, 25, 845–855. [Google Scholar] [CrossRef]
- Wesselius, T.; Heersema, D.J.; Mostert, J.P.; Heerings, M.; Admiraal-Behloul, F.; Talebian, A.; van Buchem, M.A.; De Keyser, J. A randomized crossover study of bee sting therapy for multiple sclerosis. Neurology 2005, 65, 1764–1768. [Google Scholar] [CrossRef]
- Kim, K.T.; Song, H.S. A randomized controlled double blinding study of bee venom acupuncture therapy on sprain of c-spine. J. Korean Acupunct. Moxibustion Soc. 2005, 22, 189–195. [Google Scholar]
- Mohamed, E.A.; Ewida, M.M. Efficacy of bee venom phonphoresis in treatment of chronic pelvic inflammatory diseases. Int. J. Pharm. Tech. Res. 2016, 9, 66–71. [Google Scholar]
- Nitecka-Buchta, A.; Buchta, P.; Tabeńska-Bosakowska, E.; Walczyńska-Dragoń, K.; Baron, S. Myorelaxant effect of bee venom topical skin application in patients with RDC/TMD Ia and RDC/TMD Ib: A randomized, double blinded study. Biomed. Res. Int. 2014, 2014, 296053. [Google Scholar] [CrossRef]
- An, B.J.; Song, H.S. Effect of bee venom acupuncture therapy on patients with sprain of the wrist. J. Pharmacopunct. 2006, 9, 167–171. [Google Scholar]
- Yasin, M.M.; Elhosary, E.A.; Hamada, H.A.; Yousef, A.M.; Shahin, M.; Mosaad, D. Effect of bee venom phonophoresis in obese polycystic ovarian women: A single blind randomized controlled trial. J. Appl. Pharm. Sci. 2018, 8, 159–164. [Google Scholar]
- Song, H.S. The effect of bee venom acupuncture (BVA) on acute ankle sprain: A randomized controlled trial and double blinding-pilot study. J. Pharmacopunct. 2005, 8, 11–16. [Google Scholar]
- Seo, J.W.; Park, M.J.; Sung, I.H.; Kim, N.O.; Ahn, C.K. A clinical study of bee venom acupunture therapy on the treatment of acute ankle sprain. J. Korean Acupunct. Moxibustion Soc. 2006, 23, 95–102. [Google Scholar]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, Y.E.; Doo, K.H.; Lee, J.H.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Kim, H.; Rhee, H.Y.; et al. Efficacy of combined treatment with acupuncture and bee venom acupuncture as an adjunctive treatment for parkinson’s disease. J. Altern. Complement. Med. 2018, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Mullner, J.; Meier, N.; Hesekamp, H.; van Meerbeeck, P.; Habert, M.O.; Kas, A.; Tanguy, M.L.; Mazmanian, M.; Oya, H.; et al. Bee venom for the treatment of parkinson disease-a randomized controlled clinical trial. PLoS ONE 2016, 11, e0158235. [Google Scholar]
- Park, J.A.; Lee, C.H.; Kwon, G.S.; Lee, K.A.; Jang, K.J. The effects of sweet bee venom pharmacopuncture on post-stroke hemiplegic shoulder pain. J. Korean Acupunct. Moxibustion Soc. 2011, 2, 37–47. [Google Scholar]
- Ko, C.N.; Min, I.K.; Park, S.W.; Jung, W.S.; Moon, S.K.; Park, J.M.; Cho, K.H.; Kim, Y.S.; Bae, H.S. Effectiveness of bee venom acupuncture on shoulder pain after stroke. J. Korean Orient. Med. 2007, 28, 11–24. [Google Scholar]
- Eom, J.Y.; Won, S.H.; Kwon, K.R.; Lee, H.S. Comparative study on acupuncture, bee venom acupuncture, and bee venom herbal acupuncture in the treatment of post-stroke hemiplegic shoulder pain. J. Pharmacopunct. 2006, 9, 139–154. [Google Scholar]
- Lee, D.Y.; Lee, G.K.; Yeom, S.C.; Kim, D.H.; Kim, D.J. A clinical study of bee venom acupuncture therapy on shoulder pain patients in stroke sequelae. J. Korean Acupunct. Moxibustion Soc. 2006, 23, 69–80. [Google Scholar]
- Cho, S.W.; Go, K.H.; Nam, J.H.; Kim, M.S.; Lee, S.Y.; Lee, I.S. The effectiveness of Zingiberis Rhizoma herbal acupuncture therapy and bee venom herbal acupuncture therapy on the poststroke hemiplegic shoulder pain. J. Orient. Rehab. Med. 2005, 15, 77–87. [Google Scholar]
- Lee, S.H.; Hong, S.J.; Kim, S.Y.; Yang, H.I.; Lee, J.D.; Choi, D.Y.; Lee, D.I.; Lee, Y.H. Randomized controlled double blind study of bee venom therapy on rheumatoid arthritis. J. Korean Acupunct. Moxibustion Soc. 2003, 20, 80–88. [Google Scholar]
- Chen, S.Y.; Zhou, P.; Qin, Y. Treatment of rheumatoid arthritis by bee-venom acupuncture. Zhen Ci Yan Jiu 2018, 43, 251–254. [Google Scholar] [PubMed]
- Liu, M.; Counsell, C.; Wardlaw, J.; Sandercock, P. A systematic review of randomized evidence for fibrinogen-depleting agents in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 1998, 7, 63–69. [Google Scholar] [CrossRef]
- Shetty, V.; Sriram, S.G. Effectiveness of intravenous haemocoagulase on haemorrhage control in bi-maxillary orthognathic surgery-a prospective, randomised, controlled, double-blind study. J. Craniomaxillofac. Surg. 2015, 43, 2000–2003. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, X.; Cai, H.; Xu, Z.; Lin, H. The impact of hemocoagulase for improvement of coagulation and reduction of bleeding in fracture-related hip hemiarthroplasty geriatric patients: A prospective, single-blinded, randomized, controlled study. Injury 2017, 48, 914–919. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Jin, L.; Yan, X.W. The evaluation of thrombolytic effect of snake venom antithrombus enzyme in treatment of acute myocardial infarction. Zhonghua Nei Ke Za Zhi 1994, 33, 244–247. [Google Scholar]
- Huang, D.X.; Gai, L.Y.; Wang, S.R.; Li, T.D.; Yang, T.S.; Zhi, G.; Du, L.S.; Li, L.H. Defibrase, a purified fibrinolytic protease from snake venom in acute myocardial infarction. Acta Cardiol. 1992, 47, 445–458. [Google Scholar]
- Gamba, G.; Fornasari, P.M.; Grignani, G.; Dolci, D.; Colloi, D. Haemostasis during transvesical prostatic adenomectomy. A controlled trial on the effect of drugs with antifibrinolytic and thrombin-like activities. Blut 1979, 39, 89–98. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, S.S.; Kwon, S.U.; Ha, J.H.; Suh, E.J.; Chi, H.S. Treatment of acute cerebral infarction with arginine esterase: A controlled study with heparin. Cerebrovasc. Dis. 2001, 11, 251–256. [Google Scholar] [CrossRef]
- Xu, J.; Qian, S.S.; Chen, Y.G.; Li, D.Y.; Yan, Q. Systematic review and meta-analysis of efficacy and safety of Huachansu in treating cancer-related pain. Zhongguo Zhong Yao Za Zhi 2019, 44, 2627–2636. [Google Scholar] [PubMed]
- Wu, J.; Zhang, D.; Ni, M.; Xue, J.; Wang, K.; Duan, X.; Liu, S. Effectiveness of Huachansu injection combined with chemotherapy for treatment of gastric cancer in China: A systematic review and meta-analysis. J. Tradit. Chin. Med. 2020, 40, 749–757. [Google Scholar] [PubMed]
- Zhang, D.; Wang, K.; Zheng, J.; Wu, J.; Duan, X.; Ni, M.; Liu, S.; Zhang, B.; Zhao, Y. Comparative efficacy and safety of Chinese herbal injections combined with transcatheter hepatic arterial chemoembolization in treatment of liver cancer: A bayesian network meta-analysis. J. Tradit. Chin. Med. 2020, 40, 167–187. [Google Scholar] [PubMed]
- Wang, T.; Zhang, L.; Han, L.; Liu, X.; Zhang, H.; Zhang, J.; Yu, H. Clinical effect of intravenous infusion of zoledronic acid combined with oral medication of cinobufagin in the treatment of metastatic bone tumors. Pak. J. Pharm. Sci. 2018, 31, 1609–1612. [Google Scholar] [PubMed]
- Chen, Z.; Zhai, X.F.; Su, Y.H.; Wan, X.Y.; Li, J.; Xie, J.M.; Gao, B. Clinical observation of cinobufacini injection used to treat moderate and advanced primary liver cancer. Zhong Xi Yi Jie He Xue Bao 2003, 1, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Garrett, C.R.; Shen, Y.; Liu, L.; Yang, P.; Huo, Y.; Zhao, Q.; Spelman, A.R.; Ng, C.S.; Chang, D.Z.; et al. Prospective randomised evaluation of traditional Chinese medicine combined with chemotherapy: A randomised phase II study of wild toad extract plus gemcitabine in patients with advanced pancreatic adenocarcinomas. Br. J. Cancer 2012, 107, 411–416. [Google Scholar] [CrossRef]
- Vetter, R.S.; Visscher, P.K.; Camazine, S. Mass envenomations by honey bees and wasps. West. J. Med. 1999, 170, 223–227. [Google Scholar]
- Gale, A.N. Insect-sting encephalopathy. Br. Med. J. Clin. Res. Ed. 1982, 284, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Reisman, R.E. Unusual reactions to insect stings. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 355–358. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.G.; Cho, H.J.; Bae, Y.S.; Park, K.K.; Choe, J.Y.; Chung, I.K.; Kim, M.; Yeo, J.H.; Park, K.H.; Lee, Y.S.; et al. Bee venom suppresses LPS-mediated NO/iNOS induction through inhibition of PKC-α expression. J. Ethnopharmacol. 2009, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Moon, H.J.; Li, D.X.; Gil, M.; Min, J.K.; Lee, G.; Bae, H.; Kim, S.K.; Min, B.I. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 369324. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Lee, M.H.; Choi, D.Y.; Park, D.S.; Lee, J.D. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010, 292, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Alqutub, A.N.; Masoodi, I.; Alsayari, K.; Alomair, A. Bee sting therapy-induced hepatotoxicity: A case report. World J. Hepatol. 2011, 3, 268–270. [Google Scholar] [CrossRef]
- Bae, E.J.; Son, S.B.; Seo, S.H.; Son, S.W.; Kim, I.H. A case of foreign body granuloma with skin necrosis occurring after bee sting therapy. Korean J. Dermatol. 2009, 47, 350–353. [Google Scholar]
- Cheng, Y.M.; Ren, X.H. Arrhythmia by bee sting acupuncture. J. Clin. Acupunct. Moxibustion 2004, 20, 54. [Google Scholar]
- Zhang, J.W.; Shi, D.Y.; Wang, L.Y.; Liu, R.C.; Zhang, L. Investigation of anaphylaxis by bee sting acupuncture in 9 case. Shanghai J. Acupunct. Moxibustion 1995, 3, 126. [Google Scholar]
- Jung, J.W.; Jeon, E.J.; Kim, J.W.; Choi, J.C.; Shin, J.W.; Kim, J.Y.; Park, I.W.; Choi, B.W. A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma. Immunol. Res. 2012, 4, 107–109. [Google Scholar] [CrossRef]
- Mehta, S.R.; Sashindran, V.K. Clinical features and management of snake bite. Med. J. Armed. Forces India 2002, 58, 247–249. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.R.; Mamun, S.M.H.; Rashid, R.; Rahman, M.; Ghose, A.; Sharmin, S.; Rahman, M.R.; Faiz, M.A. Anti-snake venom: Use and adverse reaction in a snake bite study clinic in Bangladesh. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 660–672. [Google Scholar] [CrossRef] [Green Version]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake venom thrombin-like enzymes: From reptilase to now. Cell Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 11 November 2020).
- Gowda, R.M.; Cohen, R.A.; Khan, I.A. Toad venom poisoning: Resemblance to digoxin toxicity and therapeutic implications. Heart 2003, 89, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Deng, L.; Cao, H.; Xu, N.; Zhang, D.; Tian, H.; Li, B.; Lu, Z.; Ye, W.; Yu, L.; et al. Screening of bufadienolides from toad venom identifies gammabufotalin as a potential anti-inflammatory Agent. Planta Med. 2020. [Google Scholar] [CrossRef]
- Banfi, F.F.; Guedes Kde, S.; Andrighetti, C.R.; Aguiar, A.C.; Debiasi, B.W.; Noronha Jda, C.; Rodrigues Dde, J.; Vieira, G.M., Jr.; Sanchez, B.A. Antiplasmodial and cytotoxic activities of toad venoms from southern amazon, Brazil. Korean J. Parasitol. 2016, 54, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Schmeda-Hirschmann, G.; Quispe, C.; Theoduloz, C.; de Sousa, P.T., Jr.; Parizotto, C. Antiproliferative activity and new argininyl bufadienolide esters from the “cururú” toad Rhinella (Bufo) schneideri. J. Ethnopharmacol. 2014, 155, 1076–1085. [Google Scholar] [CrossRef]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Warpinski, J.R.; Bush, R.K. Stinging insect allergy. J. Wilderness Med. 1990, 1, 249–257. [Google Scholar] [CrossRef]
- Chippaux, J.P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: From obvious facts to contingencies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.E. Analysis study of survey for safety and efficacy of pharmacopuncture. Aust. J. Pharm. 2010, 13, 91–102. [Google Scholar] [CrossRef]
- Ahn, U.C.; Kin, H.D.; Kim, J.H.; Rho, T.W.; Han, S.Y.; Kim, Y.K. A survey on the management status of extramural herbal dispensaries. Herb. Formula Sci. 2016, 24, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.H.; Han, J.E.; Ryu, J.Y.; Sung, A.D.M.; Park, J.Y.; Ha, I.H.; Kim, K.H.; Park, J.K.; Shin, B.C. Current status and future perspective of external herbal dispensaries preparing traditional herbal medicine in South Korea: The first national-wide survey results. BMC Complement. Med. Ther. 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]

| NO. | Pharmacopuncture | Composition of Pharmacopuncture | Amount of Preparation (vial) | Ratio of Preparation |
|---|---|---|---|---|
| 1 | Jungseong-eohyeol | Salviae Radix, Persicae Semen, Commiphora myrrha Engler, Caesalpinia sappan Linné, Olibanum, Paeoniae Radix, Corydalis yanhusuo W.T.Wang, Gardeniae Fructus | 1,373,686 | 23.45% |
| 2 | Bee venom | Bee venom | 779,931 | 13.32% |
| 3 | Hwanglyeon-haedog | Coptidis Rhizoma, Phellodendri Cortex, Scutellariae Radix, Gardeniae Fructus | 556,064 | 9.49% |
| 4 | Cheogchusin | Paeoniae Radix, Osterici seu Notopterygii Radix et Rhizoma, Araliae continentalis Radix, Cibotii Rhizoma, Eucommiae Cortex, Saposhnikoviae Radix, Acanthopanacis Cortex, Achyranthis Radix | 490,990 | 8.38% |
| 5 | Haleupagopitumgeun | Harpagophyti Radix | 272,330 | 4.65% |
| 6 | Jagyag-gamcho | Paeoniae Radix, Glycyrrhizae Radix et Rhizoma | 153,530 | 2.62% |
| 7 | Hong-hwa | Carthami Flos, Carthami tinctorii Semen | 146,380 | 2.50% |
| 8 | Jahageo | Hominis Placenta | 115,909 | 1.98% |
| 9 | Wild ginseng | Wild ginseng | 48,431 | 0.83% |
| 10 | Soyeom | Lonicerae japonicae Flos, Rehmanniae Radix, Forsythiae Fructus, Gardeniae Fructus | 20,275 | 0.35% |
| 11 | Jug-yeom | Bamboo salt | 8898 | 0.15% |
| 12 | Haeng-in | Armeniacae Semen | 7528 | 0.13% |
| 13 | Snake venom | Snake venom | 5291 | 0.09% |
| 14 | Cho-o | Aconiti Seoulense Tuber | 4433 | 0.08% |
| 15 | Yang-geumhwa | Datura metel Linné | 3530 | 0.06% |
| 16 | Chilpi | Rhus verniciflua Stokes | 3000 | 0.05% |
| 17 | Cheong-ganbohyeol | Mori Folium | 2600 | 0.04% |
| 18 | Toad venom | Toad venom | 2114 | 0.04% |
| 19 | Miso | Salmon milt | 2000 | 0.03% |
| 20 | Mahwang-cheono | Ephedrae Radix, Aconiti Tuber | 1910 | 0.03% |
| Type of Animal Venom | Treatment Diseases | Intervention (Concentration, Treatment Sessions, Amount of Venom Use) | Clinical Effects | Adverse Events | Type of Clinical Study |
|---|---|---|---|---|---|
| Bee venom | Musculoskeletal pain [17] | Bee venom acupuncture -concentration: 0.05–0.5 mg/mL -1 session: 0.01–1 mL -total 3–16 sessions: 0.05–30 mL | Positive | Skin hypersensitivity 2, itching 1, pain 2, pruritus 8, burning sensation 3 | SR |
| Bee venom | Post-stroke shoulder pain [18] | Bee venom acupuncture -concentration: 0.1–0.5 mg/mL -1 session: 0.1–1.5 mL -total 6–12 sessions: 0.9–13.5 mL | Positive | Pain 2, pruritus 8, burning sensation 3 | SR |
| Bee venom | Shoulder pain [19] | Bee venom acupuncture -concentration: 0.03–0.5 mg/mL -1 session: 0.1–1.5 mL -total 6–16 sessions: 0.6–14.8 mL | Positive | Pain 2, pruritus 8, burning sensation 3 Pruritus/local swelling/redness 30, mild, generalized swelling/aching 1 | SR |
| Bee venom | Adhesive capsulitis [20] | (A) Bee venom acupuncture -concentration: 0.1 mg/mL -1 session: 0.4 mL (first visit), 0.6 mL (second visit), 0.8 mL (third visit), 1 mL (4–16 visit) -total 16 sessions: 14.8 mL (B) Bee venom acupuncture -concentration: 0.03 mg/mL -1 session: 0.4 mL (first visit), 0.6 mL (second visit), 0.8 mL (third visit), 1 mL (4–16 visit) -total 16 sessions: 14.8 mL | Positive | Pruritus/local swelling/redness 30, mild, generalized swelling/aching 1 | RCT |
| Bee venom | Adhesive capsulitis [21] | (A) Bee venom acupuncture -concentration: 0.1 mg/mL -1 session: 0.4 mL (first visit), 0.6 mL (second visit), 0.8 mL (third visit), 1 mL (4–16 visit) -total 16 sessions: 14.8 mL (B) Bee venom acupuncture -concentration: 0.03 mg/mL -1 session: 0.4 mL (first visit), 0.6 mL (second visit), 0.8 mL (third visit), 1 mL (4–16 visit) -total 16 sessions: 14.8 mL | Positive | n.r. | RCT |
| Bee venom | Central post stroke pain [22] | Bee venom acupuncture -concentration: n.r. -1 session: 0.3 mL -total 6 sessions: 1.8 mL | Positive | No adverse events | RCT |
| Bee venom | Chronic low back pain [23] | Bee venom acupuncture -concentration: 0.05 mg/mL -1 session: 2 mL (first week), 4 mL (second week), 8 mL (third week) -total 6 sessions: 28 mL | Positive | Itching/sensation 4, headache 1, generalized myalgia 1 | RCT |
| Bee venom | Chronic low back pain [24] | Bee venom acupuncture -concentration: 0.05 mg/mL -1 session: 0.6 mL -total 8 sessions: 4.8 mL | Positive | Itching 15, skin flare 5, edema 4, rash 2 | RCT |
| Bee venom | Chronic low back pain [25] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.1 mL -total 5 sessions: 0.5 mL | Positive | Skin hypersensitivity 1 | RCT |
| Bee venom | Delayed onset muscle soreness [26] | Bee venom gel -concentration: ultrasound gel and 1 mg/mL bee venom mixed at a ratio of 9:1 -1 session: n.r. -total 3 session: n.r. | Positive | n.r. | RCT |
| Bee venom | Herniated lumbar disc [27] | Bee venom acupuncture -concentration: 0.05–0.4 mg/mL -1 session: 0.01–0.15 mL -total 7 sessions: 0.07–1.05 mL | Positive | n.r. | RCT |
| Bee venom | Herniated lumbar disc [28] | Bee venom acupuncture -concentration: 0.25 mg/mL (early stage)–0.5 mg/ mL (last stage) -1 session: 0.25 mg/mL bee venom 0.1 mL, 0.5 mg/mL bee venom 1mL -total 7 sessions: 0.25 mg/mL bee venom 3 mL, 0.5 mg/mL bee venom 30 mL | Positive | n.r. | RCT |
| Bee venom | Knee osteoarthritis [29] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.5–1 mL -total 8 sessions: 4–8 mL | Positive | n.r. | RCT |
| Bee venom | knee osteoarthritis [30] | Bee venom acupuncture -concentration: bee venom powder 1 mg and 1 mL 0.5% lidocaine were mixed -1 session: 1.2 mL(1–3 weeks), 1.5 mL (4–12) -total 12 sessions: 17.1 mL | Positive | n.r. | RCT |
| Bee venom | Multiple sclerosis [31] | Bee venom sting therapy -concentration: bee sting -1 session: maximum 20 times -total 72 sessions: maximum 1440 times | Positive | Extreme swelling 2, itching 4, flulike symptoms 5, local tenderness/swelling/redness n.r. | RCT |
| Bee venom | Neck pain [32] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.1 mL -total: n.r. | Positive | Skin hypersensitivity 1 | RCT |
| Bee venom | Pelvic inflammatory disease [33] | Bee venom gel -concentration: bee venom 20 μg/gel 1 g -1 session: n.r. -total 12 sessions: n.r. | Positive | n.r. | RCT |
| Bee venom | Temporomandibular disorder [34] | Bee venom ointment -concentration: 0.0005% -1 time: n.r. -total 42 times: n.r. | Positive | n.r. | RCT |
| Bee venom | Wrist sprain [35] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.05–10.5 mL -over 2 sessions: n.r. | Positive | n.r. | RCT |
| Bee venom | Polycystic ovary syndrome [36] | Bee venom gel -concentration: n.r. -1 session: 30–50 g -total 28 sessions: 840–1400 g | NS | n.r. | RCT |
| Bee venom | Acute ankle sprain [37] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.06 mL -total 7 sessions: 0.42 mL | Positive | Itching 1 | RCT |
| Bee venom | Acute ankle sprain [38] | Bee venom acupuncture -concentration: 0.25 mg/mL, 0.1 mg/mL -1 session: 0.25 mg/mL 0.3 mL, 0.1 mg/mL 0.2 mL -total 3 sessions: 0.25 mg/mL 0.9 mL, 0.1 mg/mL 0.6 mL | NS | n.r. | RCT |
| Bee venom | Parkinson’s disease [39] | Bee venom acupuncture -concentration: 0.05 mg/mL -1 session: 1 mL -total 16 sessions: 16 mL | Positive | Itching 1 | RCT |
| Bee venom | Parkinson’s disease [40] | Bee venom acupuncture -concentration: bee venom powder 1 mg and 20 mL normal saline were mixed -1 session: 1 mL -total 24 sessions: 24 mL | Positive | Mild pain/slight bleeding n.r., mild itching/mild swelling n.r. | RCT |
| Bee venom | Parkinson’s disease [41] | Bee venom acupuncture -concentration: bee venom 0.1 mg and 1 mL of NaCl 0.9% were mixed -1 session: 0.05 mL -total 11 sessions: 0.55 mL | NS | Redness/itching n.r., insomnia 1, nausea 3, fatigue 2, dyskinesia 1, bee venom specific IgE 18, bee venom specific IgG4 12 | RCT |
| Bee venom | Post-stroke shoulder pain [42] | Bee venom acupuncture -concentration: n.r. -1 session: 0.3–0.6 mL -total 12 sessions: 3.6–7.2 mL | Positive | n.r. | RCT |
| Bee venom | Post stroke shoulder pain [43] | Bee venom acupuncture -concentration: 0.1 mg/mL -1 session: 0.6 mL -total 6 sessions: 3.6 mL | Positive | Pain 2, pruritus 8, burning sensation 3 | RCT |
| Bee venom | Post-stroke shoulder pain [44] | Bee venom acupuncture -concentration: 0.5 mg/mL -1 session: 0.25–0.5 mL -total 12 sessions: 3–6 mL | Positive | n.r. | RCT |
| Bee venom | Post-stroke shoulder pain [45] | Bee venom acupuncture -concentration: 0.1–0.25 mg/mL -1 session: 0.1–1.5 mL -total 9 sessions: 0.9–13.5 mL | Positive | n.r. | RCT |
| Bee venom | Post-stroke shoulder pain [46] | Bee venom acupuncture -concentration: 0.05 mg/mL -1 session: 0.1 mL -total 6 sessions: 0.6 mL | NS | n.r. | RCT |
| Bee venom | Rheumatoid arthritis [47] | Bee venom acupuncture -concentration: 0.3 mg/mL -1 session: 0.2–1.0 mL -total 16 sessions: 3.2–16 mL | Positive | n.r. | RCT |
| Bee venom | Rheumatoid arthritis [48] | Bee venom sting therapy -concentration: bee sting -1 session: 5–15 times -total 24 sessions: 120–360 times | NS | Itching 60, redness 44, swelling 41, burning sensation 9, lymph node hypertrophy 2 | RCT |
| Snake venom | Acute ischemic stroke [49] | Snake venom intravenous injection -concentration:0.5–1 U/kg -1 day: 0.5–1 U/kg then variable dose to keep fibrinogen level 70–130 mg/dL -total 7–14 days | Positive | n.r. | SR |
| Snake venom | Haemorrhage control in bi-maxillary orthognathic surgery [50] | Snake venom intravenous injection -concentration: 1 U/mL aqueous solution containing 0.9% sodium chloride, 0.3% phenol, and water for injection -1session: n.r. (single bolus dose before surgery) | Positive | No adverse events | RCT |
| Snake venom | Haemorrhage control in fracture-related hip hemiarthroplasty [51] | Snake venom intravenous injection -concentration: n.r. -1 session: 1 U -total 3 sessions: 3 U, before surgery (10–15 h preoperative) and repeated in equivalent volumes at 30 min preoperative and 12 h postoperative | Positive | No adverse events | RCT |
| Snake venom | Acute myocardial infarction [52] | Snake venom intravenous injection -concentration: 7.5 U/100mL glucose solution -1 session: 7.5 U snake venom was placed in a 100 mL 5% glucose solution, and reduced to 2.5 U -total 2–14 sessions | Positive | n.r. | RCT |
| Snake venom | Acute myocardial infarction [53] | Snake venom intracoronary or intravenous injection -concentration: n.r. -1 session: n.r. (single bolus dose before surgery) | Positive | n.r. | RCT |
| Snake venom | Haemostasis during transvesical prostatic adenomectomy [54] | Snake venom intravenous injection -concentration: n.r. -1session: 1 ample -total 14 sessions: 14 samples | Positive | n.r. | RCT |
| Snake venom | Acute cerebral infarction [55] | Snake venom intravenous injection -concentration: 0.0005 U/kg -1 day: 0.0005 U/kg × 2 times/day -total 7 days: 0.007 U/kg | Positive | No adverse events | RCT |
| Toad venom | Cancer-related pain [56] | Toad venom intravenous injection -concentration: n.r. -1 session: n.r. -total 1 week–30 days sessions: n.r. | Positive | n.r. | SR |
| Toad venom | Gastric cancer [57] | Toad venom intravenous injection -concentration: n.r. -1 session: 10–50 mL -total 28–112 days: n.r. | Positive | Leukopenia 113, nausea/vomiting 56, diarrhea 28 | SR |
| Toad venom | Liver cancer [58] | Toad venom intravenous injection -concentration: n.r. -1 session: 10–30 mL -total sessions: n.r. | Positive | Nausea/vomiting 2 | SR (network meta-analysis) |
| Toad venom | Metastatic bone tumors [59] | Toad venom capsule -concentration: n.r. -1 session: 500 mg -total 36 sessions: 18 g | Positive | Fever 3, nausea and vomiting 4, diarrhea 2, muscle soreness 3 | RCT |
| Toad venom | Primary liver cancer [60] | Toad venom intravenous injection -concentration: n.r. -1 session: 20 mL toad venom was placed in a 250 mL 5% glucose solution -total 15 session: 300 mL (20 mL toad venom was placed in a 250 mL 5% glucose solution × 15 times) | Positive | n.r. | RCT |
| Toad venom | Advanced pancreatic adenocarcinomas [61] | Toad venom injection -concentration: -1session: 20 mL m−2 over 2 h -total 15 sessions: 300 mL m−2 | NS | n.r. | RCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020105
Sung S-H, Kim J-W, Han J-E, Shin B-C, Park J-K, Lee G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins. 2021; 13(2):105. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020105
Chicago/Turabian StyleSung, Soo-Hyun, Ji-Won Kim, Ji-Eun Han, Byung-Cheul Shin, Jang-Kyung Park, and Gihyun Lee. 2021. "Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication" Toxins 13, no. 2: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020105





