Reduction of Ochratoxin A during the Preparation of Porridge with Sodium Bicarbonate and Fructose
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Materials
5.2. Sample Preparation and Processing
5.3. Analyses of Color and OTA
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braaten, J.; Wood, P.; Scott, F.; Wolynetz, M.; Lowe, M.; Bradley-White, P.; Collins, M. Oat β-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur. J. Clin. Nutr. 1994, 48, 465–474. [Google Scholar]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, R.G.; Slavin, J.L. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Ramakers, J.D.; Volman, J.J.; Biörklund, M.; Önning, G.; Mensink, R.P.; Plat, J. Fecal water from ileostomic patients consuming oat β-glucan enhances immune responses in enterocytes. Mol. Nutr. Food Res. 2007, 51, 211–220. [Google Scholar] [CrossRef]
- Cappozzo, J.; Jackson, L.; Lee, H.J.; Zhou, W.; Al-Taher, F.; Zweigenbaum, J.; Ryu, D. Occurrence of ochratoxin A in infant foods in the United States. J. Food Prot. 2017, 80, 251–256. [Google Scholar] [CrossRef]
- Nguyen, K.T.N.; Ryu, D. Concentration of ochratoxin A in breakfast cereals and snacks consumed in the United States. Food Control 2014, 40, 140–144. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Significance of ochratoxin A in breakfast cereals from the United States. J. Agric. Food Chem. 2015, 63, 9404–9409. [Google Scholar] [CrossRef]
- Roscoe, V.; Lombaert, G.; Huzel, V.; Neumann, G.; Melietio, J.; Kitchen, D.; Kotello, S.; Krakalovich, T.; Trelka, R.; Scott, P. Mycotoxins in breakfast cereals from the Canadian retail market: A 3-year survey. Food Addit. Contam. 2008, 25, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rafai, P.; Bata, R.; Jakab, L.; Vanyi, A. Evaluation of mycotoxin-contaminated cereals for their use in animal feeds in Hungary. Food Addit. Contam. 2000, 17, 799–808. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1993. [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of ochratoxin A (CAS No. 303-47-9) in F344/N Rats (Gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 1989, 358, 1–146. [Google Scholar]
- Lea, T.; Steien, K.; Størmer, F.C. Mechanism of ochratoxin A-induced immunosuppression. Mycopathologia 1989, 107, 153–159. [Google Scholar] [CrossRef]
- Mayura, K.; Parker, R.; Berndt, W.; Phillips, T. Ochratoxin A-induced teratogenesis in rats: Partial protection by phenylalanine. Appl. Environ. Microbiol. 1984, 48, 1186–1188. [Google Scholar] [CrossRef] [Green Version]
- Kuruc, J.; Hegstad, J.; Lee, H.J.; Simons, K.; Ryu, D.; Wolf-Hall, C. Infestation and quantification of ochratoxigenic fungi in barley and wheat naturally contaminated with ochratoxin A. J. Food Prot. 2015, 78, 1350–1356. [Google Scholar] [CrossRef]
- Ozbey, F.; Kabak, B. Natural co-occurrence of aflatoxins and ochratoxin A in spices. Food Control 2012, 28, 354–361. [Google Scholar] [CrossRef]
- Romani, S.; Sacchetti, G.; Chaves López, C.; Pinnavaia, G.G.; Dalla Rosa, M. Screening on the occurrence of ochratoxin A in green coffee beans of different origins and types. J. Agric. Food Chem. 2000, 48, 3616–3619. [Google Scholar] [CrossRef]
- Pardo, E.; Marin, S.; Ramos, A.; Sanchis, V. Ecophysiology of ochratoxigenic Aspergillus ochraceus and Penicillium verrucosum isolates. Predictive models for fungal spoilage prevention–a review. Food Addit. Contam. 2006, 23, 398–410. [Google Scholar] [CrossRef]
- Dahal, S.; Lee, H.; Gu, K.; Ryu, D. Heat stability of ochratoxin A in an aqueous buffered model system. J. Food Prot. 2016, 79, 1748–1752. [Google Scholar] [CrossRef]
- Lee, H.J.; Dahal, S.; Perez, E.G.; Kowalski, R.J.; Ganjyal, G.M.; Ryu, D. Reduction of ochratoxin A in oat flakes by twin-screw extrusion processing. J. Food Prot. 2017, 80, 1628–1634. [Google Scholar] [CrossRef]
- Lee, H.J.; Gu, B.J.; Ganjyal, G.; Ryu, D. Reduction of ochratoxin A in direct steam injected oat-based infant cereals with baking soda. Food Control 2019, 96, 441–444. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.; Suh, H.J.; Ryu, D. Effects of explosive puffing process on the reduction of ochratoxin A in rice and oats. Food Control 2019, 95, 334–338. [Google Scholar] [CrossRef]
- Ryu, D.; Kowalski, R.J.; Ganjyal, G.; Lee, H.J. Reduction of ochratoxin A in oats and rice by twin-screw extrusion processing with baking soda. Food Control 2019, 105, 21–28. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’keefee, T.L.; Ho, Y.S.; Santillan, C.J. Occurrence of ochratoxin A contamination and detection of ochratoxigenic Aspergillus species in retail samples of dried fruits and nuts. J. Food Prot. 2015, 78, 836–842. [Google Scholar] [CrossRef]
- Van der Stegen, G.H.; Essens, P.J.; Van der Lijn, J. Effect of roasting conditions on reduction of ochratoxin A in coffee. J. Agric. Food Chem. 2001, 49, 4713–4715. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, C.; Ryu, D. Effects of baking soda and fructose in reduction of ochratoxin A in rice and oat porridge during retorting process. Food Control 2020, 107325. [Google Scholar] [CrossRef]
- Katofsky, R.E. The Production of Fluid Fuels from Biomass; Princeton University: Princeton, NJ, USA, 1993. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Al-Taher, F.; Cappozzo, J.; Zweigenbaum, J.; Lee, H.J.; Jackson, L.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the US market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Abrunhosa, L.; Paterson, R.R.; Venâncio, A. Biodegradation of ochratoxin A for food and feed decontamination. Toxins 2010, 2, 1078–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castegnaro, M.; Tozlovanu, M.; Wild, C.; Molinié, A.; Sylla, A.; Pfohl-Leszkowicz, A. Advantages and drawbacks of immunoaffinity columns in analysis of mycotoxins in food. Mol. Nutr. Food Res. 2006, 50, 480–487. [Google Scholar] [CrossRef] [PubMed]
- FDA. CFR—Code of Federal Regulations Title 21. 2017. Available online: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1736 (accessed on 1 February 2021).
- Voss, K.A.; Bullerman, L.B.; Bianchini, A.; Hanna, M.A.; Ryu, D. Reduced toxicity of fumonisin B1 in corn grits by single-screw extrusion. J. Food Prot. 2008, 71, 2036–2041. [Google Scholar] [CrossRef]
- Jackson, L.S.; Jablonski, J.; Bullerman, L.B.; Bianchini, A.; Hanna, M.A.; Voss, K.A.; Hollub, A.D.; Ryu, D. Reduction of fumonisin B1 in corn grits by twin-screw extrusion. J. Food Sci. 2011, 76, T150–T155. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Ryu, D.; Lee, H.J. Ochratoxin A and its reaction products affected by sugars during heat processing. Food Chem. 2021, 348, 129038. [Google Scholar] [CrossRef]
- Trenk, H.L.; Butz, M.E.; Chu, F.S. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl. Environ. Microbiol. 1971, 21, 1032–1035. [Google Scholar] [CrossRef]
- Berk, Z. Chapter 1—Physical Properties of Food Materials; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 February 2021).
- Woo, K.S.; Kim, H.Y.; Hwang, I.G.; Lee, S.H.; Jeong, H.S. Characteristics of the thermal degradation of glucose and maltose solutions. Prev. Nutr. Food Sci. 2015, 20, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salak Asghari, F.; Yoshida, H. Acid-catalyzed production of 5-hydroxymethyl furfural from D-fructose in subcritical water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Fagerson, I.S. Thermal degradation of carbohydrates; a review. J. Agric. Food Chem. 1969, 17, 747–750. [Google Scholar] [CrossRef]
- Khajavi, S.H.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Kinetics on sucrose decomposition in subcritical water. LWT-Food Sci. Technol. 2005, 38, 297–302. [Google Scholar] [CrossRef]
| Sample | Additives | L1 | A2 | B3 | ΔE4 | |
|---|---|---|---|---|---|---|
| Type | Amount (%, w/w) 5 | |||||
| Rice-based porridge | No additives | 89.1 ± 1.4 a,6 | −0.7 ± 0.1 a | 8.6 ± 0.5 c | 1.9 ± 1.6 b | |
| Sodium bicarbonate | 0.5 | 85.9 ± 0.7 c | −2.1 ± 0.4 c | 15.5 ± 1.0 a | 6.4 ± 1.7 a | |
| 1 | 86.7 ± 0.3 bc | −3.1 ± 0.2 d | 17.2 ± 1.7 a | 8.0 ± 1.6 a | ||
| Fructose | 0.5 | 89.2 ± 0.6 a | −0.8 ± 0.1 a | 8.9 ± 0.6 c | 1.1 ± 0.5 b | |
| 1 | 87.8 ± 0.9 abc | −0.8 ± 0.1 a | 9.7 ± 1.0 bc | 1.2 ± 1.3 b | ||
| 5 | 87.1 ± 0.5 abc | −0.9 ± 0.2 a | 10.8 ± 1.1 bc | 1.9 ± 1.3 b | ||
| 10 | 88.7 ± 0.3 ab | −0.6 ± 0.0 a | 10.6 ± 1.0 bc | 0.9 ± 0.5 b | ||
| Sodium bicarbonate + fructose | 0.5 + 0.5 | 87.2 ± 0.6 abc | −1.5 ± 0.1 b | 12.3 ± 1.0 b | 3.0 ± 0.5 b | |
| Oat-based porridge | No additives | 72.3 ± 3.4 a | 3.1 ± 0.6 ab | 19.6 ± 2.3 NS | 3.1 ± 1.6 bc | |
| Sodium bicarbonate | 0.5 | 67.5 ± 2.3 ab | 2.7 ± 0.2 b | 18.5 ± 0.8 | 4.2 ± 2.3 bc | |
| 1 | 61.5 ± 1.3 c | 1.7 ± 0.2 c | 19.7 ± 0.8 | 10.2 ± 1.2 a | ||
| Fructose | 0.5 | 72.9 ± 1.2 a | 2.8 ± 0.2 b | 18.4 ± 0.2 | 1.5 ± 1.2 c | |
| 1 | 70.6 ± 1.6 ab | 3.2 ± 0.2 ab | 18.4 ± 0.4 | 1.3 ± 0.9 c | ||
| 5 | 67.6 ± 1.6 ab | 3.8 ± 0.2 a | 20.1 ± 0.5 | 4.2 ± 1.8 bc | ||
| 10 | 71.2 ± 1.2 a | 3.2 ± 0.2 ab | 19.0 ± 0.2 | 1.0 ± 0.8 c | ||
| Combination of sodium bicarbonate and fructose | 0.5 + 0.5 | 65.7 ± 1.7 bc | 2.8 ± 0.1 b | 18.1 ± 0.1 | 5.9 ± 1.4 b | |
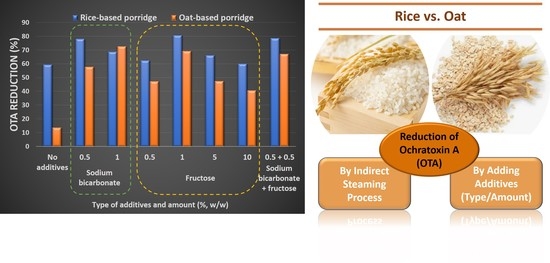
| Additives | OTA Reduction (%) 1 | ||
|---|---|---|---|
| Type | Amount (%, w/w) 2 | Rice-Based Porridge | Oat-Based Porridge |
| No additives | 59.4 ± 1.9 c,3 | 13.6 ± 1.2 e,* | |
| Sodium bicarbonate | 0.5 | 78.1 ± 1.8 a | 57.7 ± 6.5 bc,* |
| 1 | 68.7 ± 0.5 b | 72.6 ± 2.0 a | |
| Fructose | 0.5 | 62.5 ± 1.2 bc | 47.3 ± 4.3 cd,* |
| 1 | 80.7 ± 3.1 a | 69.3 ± 5.0 ab,* | |
| 5 | 66.1 ± 1.2 bc | 47.5 ± 7.8 cd,* | |
| 10 | 60.0 ± 5.3 c | 40.7 ± 1.2 d,* | |
| Combination of sodium bicarbonate and fructose | 0.5 + 0.5 | 78.6 ± 1.0 a | 67.2 ± 5.9 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Li, S.; Gu, K.; Ryu, D. Reduction of Ochratoxin A during the Preparation of Porridge with Sodium Bicarbonate and Fructose. Toxins 2021, 13, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030224
Lee HJ, Li S, Gu K, Ryu D. Reduction of Ochratoxin A during the Preparation of Porridge with Sodium Bicarbonate and Fructose. Toxins. 2021; 13(3):224. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030224
Chicago/Turabian StyleLee, Hyun Jung, Shufang Li, Kejia Gu, and Dojin Ryu. 2021. "Reduction of Ochratoxin A during the Preparation of Porridge with Sodium Bicarbonate and Fructose" Toxins 13, no. 3: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030224