New Isolated Metschnikowia pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium expansum and Patulin Accumulation
Abstract
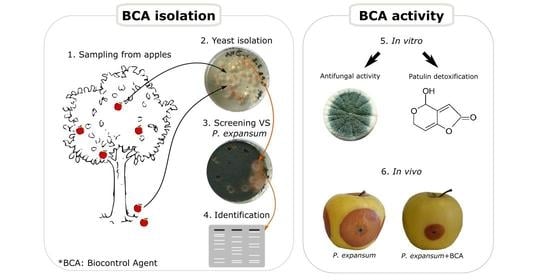
:1. Introduction
2. Results
2.1. Screening for Antagonistic Yeasts
2.2. Identification and Characterization of Antagonistic Yeast
2.3. Comparison of In Vitro Biocontrol Efficacy of Different Concentrations of Antagonistic Yeasts
2.4. Biodegradation of Patulin by Antagonistic Yeasts in Liquid Medium
2.4.1. Check of Patulin Impact on Yeast Cell Viability
2.4.2. Patulin Reduction by Antagonistic Yeasts
2.5. Biocontrol Activity of Y29 Applied on Apples
2.6. Patulin Quantification on Apples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation and Qualitative Confrontation Test
5.2. Molecular Analysis
5.2.1. Identification
Amplification Reactions
Sequence Analysis of the ITS1-5.8S-ITS2 Region
5.2.2. Characterization by PCR Fingerprinting Assay
5.3. Antifungal Activity of Yeasts
5.4. Biodegradation of Patulin by Antagonistic Yeasts
5.4.1. Microorganisms and Culture Conditions
5.4.2. Influence of Patulin on Yeast Cell Viability
5.4.3. HPLC MS/MS Analysis
5.4.4. Patulin Cell Adsorption or Absorption
5.5. Antifungal Activity of the Strain Y29 Applied on Apples
5.5.1. Strain Y29 Application and Antifungal Activity
5.5.2. Yeasts Viability after Drying
5.6. Patulin Quantification on Apple
5.6.1. Patulin Extraction
5.6.2. HPLC MS/MS Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Preventing post-harvest losses in the apple supply chain in Lebanon. In Guide: Preventing Post-Harvest Losses in the Apple Supply Chain in Lebanon; FAO: Lebnon, Beirut, 2018; p. 5. [Google Scholar]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Demirel, R.; Sariozlu, N.Y.; Ilhan, S. Polymerase chain reaction (PCR) identification of terverticillate penicillium species isolated from agricultural soils in Eskisehir Province. Braz. Arch. Biol. Technol. 2013, 56, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Gutarowska, B.; Skora, J.; Zduniak, K.; Rembisz, D. Analysis of the sensitivity of microorganisms contaminating museums and archives to silver nanoparticles. Int. Biodeterior. Biodegrad. 2012, 68, 7–17. [Google Scholar] [CrossRef]
- Van De Perre, E.; Jacxsens, L.; Van Der Hauwaert, W.; Haesaert, I.; De Meulenaer, B. Screening for the presence of patulin in molded fresh produce and evaluation of its stability in the production of tomato products. J. Agric. Food Chem. 2014, 62, 304–309. [Google Scholar] [CrossRef]
- Pattono, D.; Grosso, A.; Stocco, P.P.; Pazzi, M.; Zeppa, G. Survey of the presence of patulin and ochratoxin A in traditional semi-hard cheeses. Food Control 2013, 33, 54–57. [Google Scholar] [CrossRef]
- Vansteelandt, M.; Kerzaon, I.; Blanchet, E.; Fossi Tankoua, O.; Robiou Du Pont, T.; Joubert, Y.; Monteau, F.; Le Bizec, B.; Frisvad, J.C.; Pouchus, Y.F.; et al. Patulin and secondary metabolite production by marine-derived Penicillium strains. Fungal Biol. 2012, 116, 954–961. [Google Scholar] [CrossRef]
- Geiger, M.; Guitton, Y.; Vansteelandt, M.; Kerzaon, I.; Blanchet, E.; Robiou du Pont, T.; Frisvad, J.C.; Hess, P.; Pouchus, Y.F.; Grovel, O. Cytotoxicity and mycotoxin production of shellfish-derived Penicillium spp., a risk for shellfish consumers. Lett. Appl. Microbiol. 2013, 57, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Tournas, V.H.; Uppal Memon, S. Internal contamination and spoilage of harvested apples by patulin-producing and other toxigenic fungi. Int. J. Food Microbiol. 2009, 133, 206–209. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Res. 2010, 26, 257–265. [Google Scholar] [CrossRef]
- Tannous, J.; El Khoury, R.; Snini, S.P.; Lippi, Y.; El Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation EC 1881/2006, setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, No 1881, 26.
- Dong, X.; Jiang, W.; Li, C.; Ma, N.; Xu, Y.; Meng, X. Patulin biodegradation by marine yeast Kodameae ohmeri. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 352–360. [Google Scholar] [CrossRef]
- Mercier, J.; Smilanick, J.L. Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol. Control 2005, 32, 401–407. [Google Scholar] [CrossRef]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Chanchaichaovivat, A.; Ruenwongsa, P.; Panijpan, B. Screening and identification of yeast strains from fruits and vegetables: Potential for biological control of postharvest chilli anthracnose (Colletotrichum capsici). Biol. Control 2007, 42, 326–335. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Unraveling the mechanisms used by antagonistic yeast To control postharvest pathogens on fruit. In Proceedings of the III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability, Bari, Italy, 7 June 2015; Volume 1144, pp. 63–70. [Google Scholar]
- Irtwange, S.V. Application of Biological Control Agents in Pre-and Postharvest Operations; Agricultural Engineering International. CIGR J. 2006, 3, 3. [Google Scholar]
- Muccilli, S.; Restuccia, C. Bioprotective Role of Yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [Green Version]
- Manso, T.; Nunes, C. Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol. Technol. 2011, 61, 64–71. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Cunha, S.C.; Faria, M.A.; Pereira, V.L.; Oliveira, T.M.; Lima, A.C.; Pinto, E. Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food Control 2014, 44, 185–190. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Yang, Q.; Ren, R. Efficacy of Pichia caribbica in controlling blue mold rot and patulin degradation in apples. Int. J. Food Microbiol. 2013, 162, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Castoria, R.; Mannina, L.; Durán-Patrón, R.; Maffei, F.; Sobolev, A.P.; De Felice, D.V.; Wright, S.A. Conversion of the mycotoxin patulin to the less toxic desoxypatulinic acid by the biocontrol yeast Rhodosporidium kratochvilovae strain LS11. J. Agric. Food Chem. 2011, 59, 11571–11578. [Google Scholar] [CrossRef] [Green Version]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Al Riachy, R.; Strub, C.; Durand, N.; Guibert, B.; Guichard, H.; Constancias, F.; Chochois, V.; Lopez-Lauri, F.; Fontana, A.; Schorr-Galindo, S. Microbiome Status of Cider-Apples, from Orchard to Processing, with a Special Focus on Penicillium expansum Occurrence and Patulin Contamination. J. Fungi 2021, 7, 244. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Tworkoski, T.J.; Kurtzman, C.P. Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology 2001, 91, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, O.; Prillinger, H. Analysis of yeast isolates related to Metschnikowia pulcherrima using the partial sequences of the large subunit rDNA and the actin gene; Description of Metschnikowia andauensissp. nov. Syst. Appl. Microbiol. 2005, 28, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.R. Distribution of yeasts and yeast-like organisms from aerial surfaces of developing apples and grapes. In Microbiology of Aerial Plant Surfaces; Academic Press: Cambridge, MA, USA, 1976; pp. 325–359. [Google Scholar]
- Spadaro, D.; Sabetta, W.; Acquadro, A.; Portis, E.; Garibaldi, A.; Gullino, M.L. Use of AFLP for differentiation of Metschnikowia pulcherrima strains for postharvest disease biological control. Microbiol. Res. 2008, 163, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Lorè, A.; Garibaldi, A.; Gullino, M.L. A new strain of Metschnikowia fructicola for postharvest control of Penicillium expansum and patulin accumulation on four cultivars of apple. Postharvest Biol. Technol. 2013, 75, 1–8. [Google Scholar] [CrossRef]
- Miller, M.W.; Phaff, H.J. Metschnikowia Kamienski. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 1998; pp. 256–267. [Google Scholar]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2019, 112, 1425–1445. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, L.; Li, S.; Gao, X.; Wu, N.; Zhao, Y.; Sun, W. Control of postharvest grey spot rot of loquat fruit with Metschnikowia pulcherrima E1 and potential mechanisms of action. Biol. Control 2021, 152, 104406. [Google Scholar] [CrossRef]
- Pawlikowska, E.; Kręgiel, D. Enzymatic Profiles and Antimicrobial Activity of the Yeast Metschnikowia Pulcherrima. Acta Innov. 2017, 6, 1–7. [Google Scholar]
- Saravanakumar, D.; Ciavorella, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 2008, 49, 121–128. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Antilisterial properties of PVOH-based films embedded with Lactococcus lactis subsp. lactis. Food Hydrocoll. 2019, 87, 214–220. [Google Scholar] [CrossRef]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible Films and Coatings as Carriers of Living Microorganisms: A New Strategy Towards Biopreservation and Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barad, S.; Sionov, E.; Prusky, D. Role of patulin in post-harvest diseases. Fungal Biol. Rev. 2016, 30, 24–32. [Google Scholar] [CrossRef]
- Li, X.; Tang, H.; Yang, C.; Meng, X.; Liu, B. Detoxification of mycotoxin patulin by the yeast Rhodotorula mucilaginosa. Food Control 2019, 96, 47–52. [Google Scholar] [CrossRef]
- Zhu, R.; Feussner, K.; Wu, T.; Yan, F.; Karlovsky, P.; Zheng, X. Detoxification of mycotoxin patulin by the yeast Rhodosporidium paludigenum. Food Chem. 2015, 179, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O.; Long, M.T. Fate of patulin in the presence of the yeast Saccharomyces cerevisiae. Food Addit. Contam. 2002, 19, 387–399. [Google Scholar] [CrossRef]
- Coelho, A.R.; Celli, M.G.; Ono, E.Y.S.; Wosiacki, G.; Hoffmann, F.L.; Pagnocca, F.C.; Hirooka, E.Y. Penicillium expansum versus antagonist yeasts and patulin degradation in vitro. Braz. Arch. Biol. Technol. 2007, 50, 725–733. [Google Scholar] [CrossRef]
- Diao, E.; Hou, H.; Hu, W.; Dong, H.; Li, X. Removing and detoxifying methods of patulin: A review. Trends Food Sci. Technol. 2018, 81, 139–145. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Potential of two Metschnikowia pulcherrima (yeast) strains for in vitro biodegradation of patulin. J. Food Prot. 2011, 74, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.; Marín, S.; Ramos, A.J.; Sanchis, V. Influence of post-harvest technologies applied during cold storage of apples in Penicillium expansum growth and patulin accumulation: A review. Food Control 2010, 21, 953–962. [Google Scholar] [CrossRef]
- Nguyen Van Long, N.; Vasseur, V.; Coroller, L.; Dantigny, P.; Le Panse, S.; Weill, A.; Mounier, J.; Rigalma, K. Temperature, water activity and pH during conidia production affect the physiological state and germination time of Penicillium species. Int. J. Food Microbiol. 2017, 241, 151–160. [Google Scholar] [CrossRef]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Keller, N.P.; Atoui, A.; El Khoury, A.; Lteif, R.; Oswald, I.P.; Puel, O. Secondary metabolism in Penicillium expansum: Emphasis on recent advances in patulin research. Crit. Rev. Food Sci. Nutr. 2018, 58, 2082–2098. [Google Scholar] [CrossRef] [PubMed]
- Querol, A.; Barrio, E.; Huerta, T.; Ramon, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peŕez-Través, L.; Lopes, C.A.; Querol, A.; Barrio, E. On the complexity of the Saccharomyces bayanus taxon: Hybridization and potential hybrid speciation. PLoS ONE 2014, 9, e93729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canelas, A.B.; ten Pierick, A.; Ras, C.; Seifar, R.M.; van Dam, J.C.; van Gulik, W.M.; Heijnen, J.J. Quantitative Evaluation of Intracellular Metabolite Extraction Techniques for Yeast Metabolomics. Anal. Chem. 2009, 81, 7379–7389. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana, A.; Strub, C.; Schorr-Galindo, S. New Isolated Metschnikowia pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium expansum and Patulin Accumulation. Toxins 2021, 13, 397. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13060397
Settier-Ramírez L, López-Carballo G, Hernández-Muñoz P, Fontana A, Strub C, Schorr-Galindo S. New Isolated Metschnikowia pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium expansum and Patulin Accumulation. Toxins. 2021; 13(6):397. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13060397
Chicago/Turabian StyleSettier-Ramírez, Laura, Gracia López-Carballo, Pilar Hernández-Muñoz, Angélique Fontana, Caroline Strub, and Sabine Schorr-Galindo. 2021. "New Isolated Metschnikowia pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium expansum and Patulin Accumulation" Toxins 13, no. 6: 397. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13060397