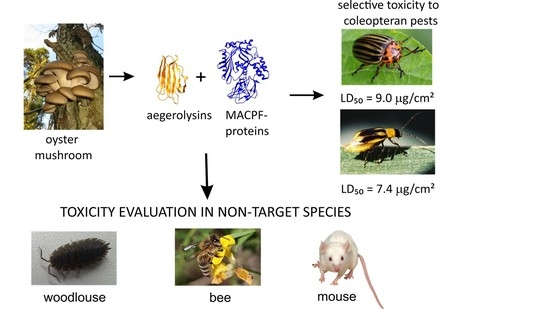
Effects of Bioinsecticidal Aegerolysin-Based Cytolytic Complexes on Non-Target Organisms
Abstract
:1. Introduction
2. Results
2.1. Toxicity Tests with Woodlice (Porcellio scaber)
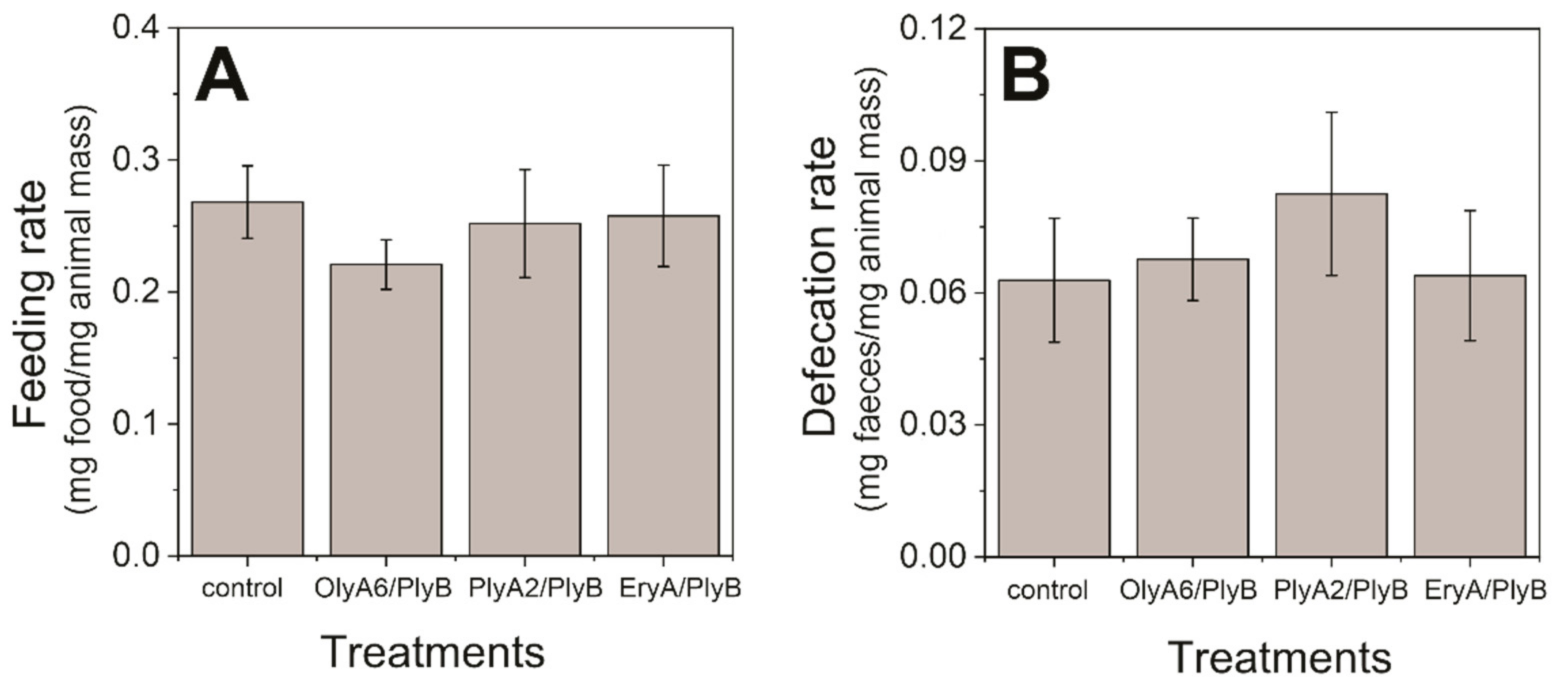
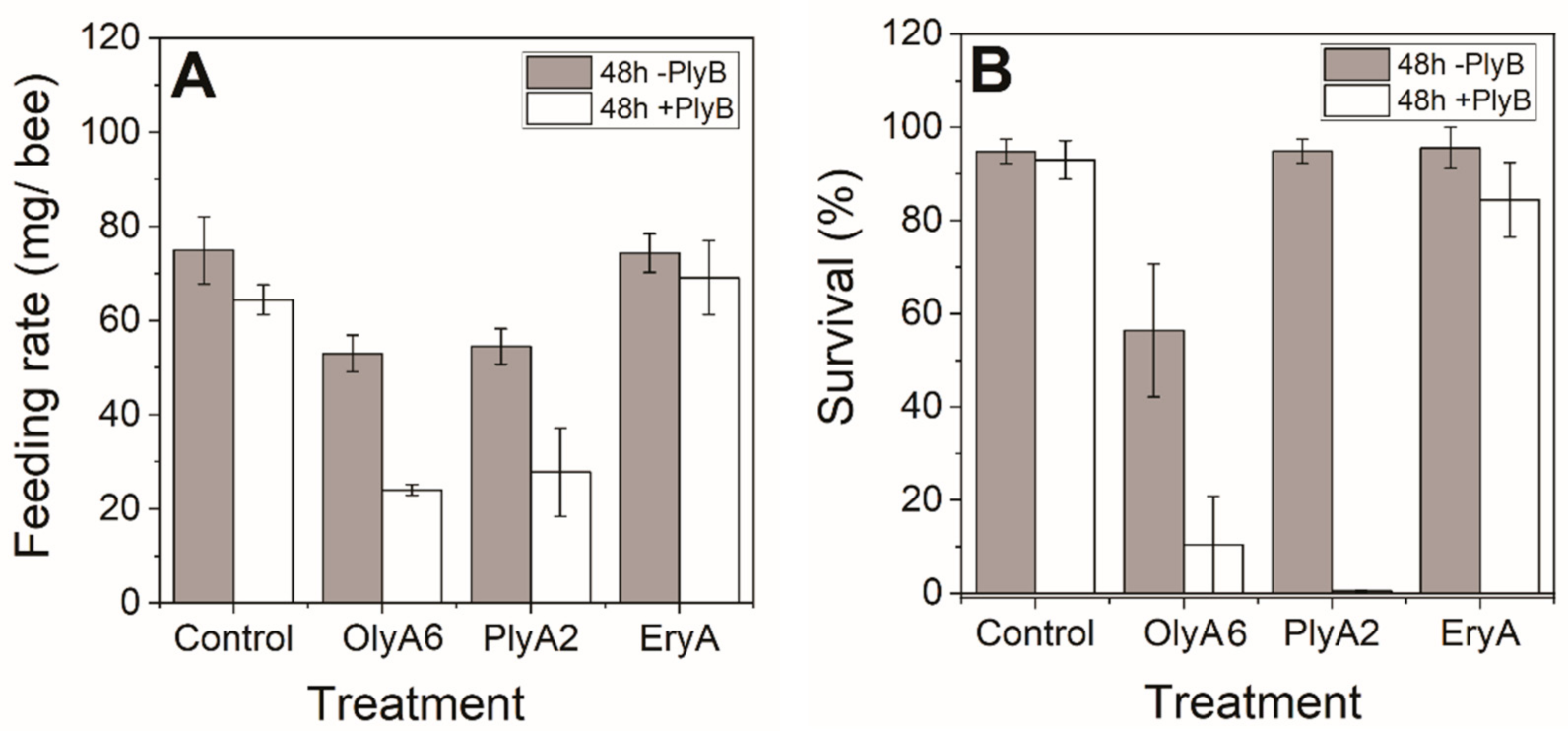
2.2. Toxicity Tests with Honey Bees (Apis mellifera carnica)
2.2.1. Toxicities of Aegerolysin and Their Mixtures with PlyB
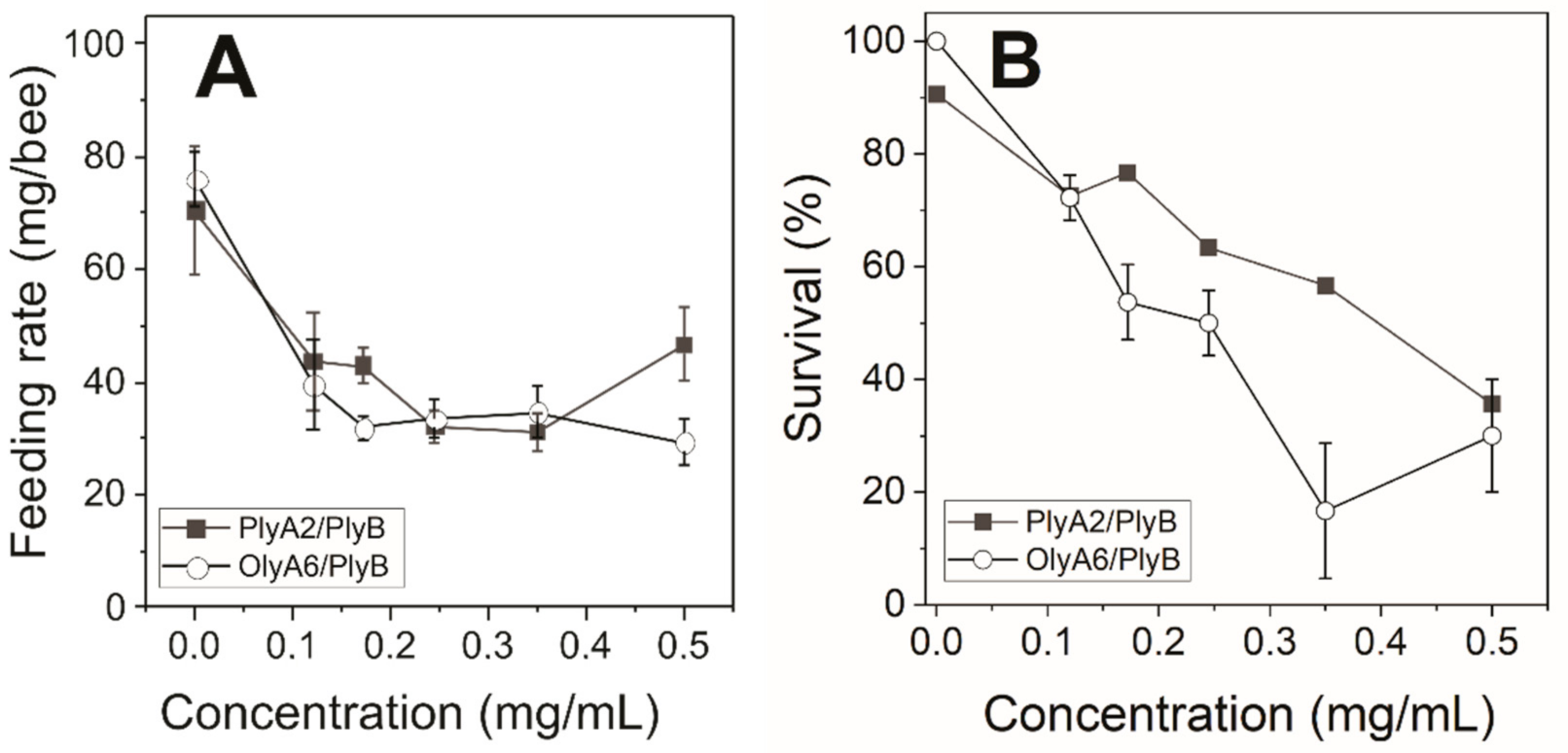
2.2.2. Dose–Response Toxicity Testing of OlyA6/PlyB and PlyA2/PlyB
2.3. Determination of Acute Toxicity of EryA/PlyB in Mice
2.3.1. Body and Organ Mass
2.3.2. Histological Evaluation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Animals
5.3. Toxicity Testing
5.3.1. Woodlice (Porcellio scaber)
5.3.2. Honeybees (Apis mellifera carnica)
5.3.3. Mice (Mus musculus)
5.3.4. Histological Analysis of the Mice Organs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panevska, A.; Hodnik, V.; Skočaj, M.; Novak, M.; Modic, Š.; Pavlic, I.; Podržaj, S.; Zarić, M.; Resnik, N.; Maček, P.; et al. Pore-forming protein complexes from Pleurotus mushrooms kill western corn rootworm and Colorado potato beetle through targeting membrane ceramide phosphoethanolamine. Sci. Rep. 2019, 9, 5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butala, M.; Novak, M.; Kraševec, N.; Skočaj, M.; Veranič, P.; Maček, P.; Sepčić, K. Aegerolysins: Lipid-binding proteins with versatile functions. Semin. Cell Dev. Biol. 2017, 72, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Noguchi, K.; Mimuro, H.; Ukaji, F.; Ito, K.; Sugawara-Tomita, N.; Hashimoto, Y. Pleurotolysin, a novel sphingomyelin-specific two-component cytolysin from the edible mushroom Pleurotus ostreatus, assembles into a transmembrane pore complex. J. Biol. Chem. 2004, 279, 26975–26982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, K.; Leonardi, A.; Mikelj, M.; Skočaj, M.; Wohlschlager, T.; Künzler, M.; Aebi, M.; Narat, M.; Križaj, I.; Anderluh, G.; et al. Membrane cholesterol and sphingomyelin, and ostreolysin A are obligatory for pore-formation by a MACPF/CDC-like pore-forming protein, pleurotolysin B. Biochimie 2013, 95, 1855–1864. [Google Scholar] [CrossRef]
- Lukoyanova, N.; Kondos, S.C.; Farabella, I.; Law, R.H.P.; Reboul, C.F.; Caradoc-Davies, T.T.; Spicer, B.A.; Kleifeld, O.; Traore, D.A.K.; Ekkel, S.M.; et al. Conformational changes during pore formation by the perforin-related protein pleurotolysin. PLoS Biol. 2015, 13, e1002049. [Google Scholar] [CrossRef] [Green Version]
- Bhat, H.B.; Kishimoto, T.; Abe, M.; Makino, A.; Inaba, T.; Murate, M.; Dohmae, N.; Kurahashi, A.; Nishibori, K.; Fujimori, F.; et al. Binding of a pleurotolysin ortholog from Pleurotus eryngii to sphingomyelin and cholesterol-rich membrane domains. J. Lipid Res. 2013, 54, 2933–2943. [Google Scholar] [CrossRef] [Green Version]
- Bhat, H.B.; Ishitsuka, R.; Inaba, T.; Murate, M.; Abe, M.; Makino, A.; Kohoyama-Koganeya, A.; Kurahashi, A.; Kishimoto, T.; Tahara, M.; et al. Evaluation of aegerolysins as novel tools to detect and visualize ceramide phosphoethanolamine, a major sphingolipid in invertebrates. FASEB J. 2015, 29, 3920–3934. [Google Scholar] [CrossRef] [Green Version]
- Panevska, A.; Skočaj, M.; Križaj, I.; Maček, P.; Sepčić, K. Ceramide phosphoethanolamine, an enigmatic cellular membrane sphingolipid. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1284–1292. [Google Scholar] [CrossRef]
- Kishimoto, T.; Ishitsuka, R.; Kobayashi, T. Detectors for evaluating the cellular landscape of sphingomyelin- and cholesterol-rich membrane domains. Biochim. Biophys. Acta 2016, 1861, 812–829. [Google Scholar] [CrossRef]
- Novak, M.; Krpan, T.; Panevska, A.; Shewell, L.K.; Day, C.J.; Jennings, M.P.; Guella, G.; Sepčić, K. Binding specificity of ostreolysin A6 towards Sf9 insect cell lipids. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183307. [Google Scholar] [CrossRef]
- Grafius, E.; Douches, D. Progress in Biological Control; Romeis, J., Shelton, A.M., Kennedy, G.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 195–221. [Google Scholar]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, L. Advances in Insect Physiology; Tarlochan, S.D., Sarjeet, S.G., Eds.; Academic Press: London, UK, 2014; pp. 39–87. [Google Scholar]
- Kamionskaya, A.M.; Kuznetsov, B.B.; Ismailov, V.Y.; Nadiktal, V.D.; Skryabin, K.G. Genetically transforming Russian potato cultivars for resistance to Colorado beetle. Cloning Transgenes 2012, 1, 101. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.; Ji, X.; Yang, J.; Liang, L.; Si, H.; Wu, J.; Zhang, N.; Wang, D. Transgenic potato plants expressing cry3A gene confer resistance to Colorado potato beetle. Comptes Rendus Biol. 2015, 338, 443–450. [Google Scholar] [CrossRef]
- USEPA. Biopesticides Registration Action Document. Bacillus thuringiensis Cry3Bb1 Protein and the Genetic Material Necessary for Its Production in MON 863 and MON 88017 Corns. 2010. Available online: http://www.epa.gov/pesticides/biopesticides/pips/cry3bb1-brad.pdf (accessed on 29 June 2021).
- Moellenbeck, D.J.; Peters, M.L.; Bing, J.W.; Rouse, J.R.; Higgins, L.S.; Sims, L.; Nevshemal, T.; Marshall, L.; Ellis, R.T.; Bystrak, P.G.; et al. Insecticidal proteins from Bacillus thuringiensis protect corn from corn rootworms. Nat. Biotechnol. 2001, 19, 668–672. [Google Scholar] [CrossRef]
- Kelker, M.S.; Berry, C.; Evans, S.L.; Pai, R.; McCaskill, D.G.; Wand, N.X.; Russell, J.C.; Baker, M.D.; Yand, C.; Pflugrath, J.W.; et al. Structural and biophysical characterization of Bacillus thuringiensis insecticidal proteins Cry34Ab1 and Cry35Ab1. PLoS ONE 2014, 9, e112555. [Google Scholar] [CrossRef]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [Green Version]
- Ferré, J.; van Rie, J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002, 47, 501–533. [Google Scholar] [CrossRef]
- De Almeida Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Shretstha, R.B.; Kropf, A.L.; St. Clair, C.R.; Brenizer, B.D. Field-evolved resistance by western corn rootworm to Cry34/35Ab1 and other Bacillus thuringiensis traits in transgenic maize. Pest Manag. Sci. 2020, 76, 268–276. [Google Scholar]
- Kaiser-Alexnat, R. Protease activities in the midgut of western corn rootworm (Diabrotica virgifera virgifera LeConte). J. Invertebr. Pathol. 2009, 100, 169–174. [Google Scholar] [CrossRef]
- Felton, G.W.; Workman, J.; Duffey, S.S. Avoidance of antinutritive plant defense: Role of midgut pH in Colorado potato beetle. J. Chem. Ecol. 1992, 18, 571–583. [Google Scholar] [CrossRef]
- Berne, S.; Sepčić, K.; Anderluh, G.; Turk, T.; Maček, P.; Ulrih, N.P. Effect of pH on the pore forming activity and conformational stability of ostreolysin, a lipid raft-binding protein from the edible mushroom Pleurotus ostreatus. Biochemistry 2005, 44, 11137–11147. [Google Scholar] [CrossRef] [PubMed]
- Žužek, M.C.; Maček, P.; Sepčić, K.; Cestnik, V.; Frangež, R. Toxic and lethal effects of ostreolysin, a cytolytic protein from edible oyster mushroom (Pleurotus ostreatus), in rodents. Toxicon 2006, 48, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; Loureiro, S.; Zidar, P. Terrestrial isopods as model organisms in soil ecotoxicology: A review. Zookeys 2018, 801, 127–162. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, 80–95. [Google Scholar] [CrossRef]
- Wang, M.J.; Chen, F.; Lau, J.T.Y.; Hu, Y.P. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017, 8, e2805. [Google Scholar] [CrossRef]
- Juntes, P.; Rebolj, K.; Sepčić, K.; Maček, P.; Žužek, M.C.; Cestnik, V.; Frangež, R. Ostreolysin induces sustained contraction of porcine coronary arteries and endothelial dysfunction in middle- and large-sized vessels. Toxicon 2009, 54, 784–792. [Google Scholar] [CrossRef]
- Romeis, J.; Bartsch, D.; Bigler, F.; Candolfi, M.P.; Gielkens, M.M.; Hartley, S.E.; Hellmich, R.L.; Huesing, J.E.; Jepson, P.C.; Layton, R.; et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 2008, 26, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Juberg, D.R.; Herman, R.A.; Thomas, J.; Brooks, K.J.; Delaney, B. Acute and repeated dose (28 day) mouse oral toxicology studies with Cry34Ab1 and Cry35Ab1 Bt proteins used in coleopteran resistant DAS-59122-7 corn. Regul. Toxicol. Pharmacol. 2009, 54, 154–163. [Google Scholar] [CrossRef]
- EPA—U.S. Environmental Protection Agency. Biopesticides Registration Action Document. Bacillus thuringiensis Cry34Ab1 and Cry35Ab1 Proteins and the Genetic Material Necessary for Their Production (PHP17662 T-DNA) in Event DAS-59122-7 Corn (OECD Unique Identifier: DAS-59122-7), September 2010. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/cry3435ab1-brad.pdf (accessed on 29 June 2021).
- CERA. A Review of the Environmental Safety of the Cry34Ab1 and Cry35Ab1 Proteins; Center for Environmental Risk Assessment (CERA), ILSI Research Foundation: Washington, DC, USA, 2013. [Google Scholar]
- Malley, L.A.; Everds, N.E.; Reynolds, J.; Mann, P.C.; Lamb, I.; Rood, T.; Schmidt, J.; Layton, R.J.; Prochaska, L.M.; Hinds, M.; et al. Subchronic feeding study of DAS-59122-7 maize grain in Sprague-Dawley rats. Food Chem. Toxicol. 2007, 45, 1277–1292. [Google Scholar] [CrossRef]
- Zimmer, M.; Brune, A. Physiological properties of the gut lumen of terrestrial isopods (Isopoda: Oniscidea): Adaptive to digesting lignocellulose? J. Comp. Physiol. B 2005, 175, 275–283. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- O’Connor, J.; Polito, A.; Monroe, R.; Sweeley, C.; Bleber, L. Characterization of invertebrate sphingolipid bases: Occurrence of eicosasphinga-4, 11-dienine and eicosasphing-11-enine in scorpion. Biochim. Biophys. Acta 1970, 202, 195–197. [Google Scholar] [CrossRef]
- Mohr, K.I.; Tebbe, C.C. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 2006, 8, 258–272. [Google Scholar] [CrossRef]
- Skočaj, M.; Resnik, N.; Grundner, M.; Ota, K.; Rojko, N.; Hodnik, V.; Anderluh, G.; Sobota, A.; Maček, P.; Veranič, P.; et al. Tracking cholesterol/sphingomyelin-rich membrane domains with the ostreolysin A-mCherry protein. PLoS ONE 2014, 9, e92783. [Google Scholar] [CrossRef] [Green Version]
- Skočaj, M.; Yu, Y.; Grundner, M.; Resnik, N.; Bedina Zavec, A.; Leonardi, A.; Križaj, I.; Guella, G.; Maček, P.; Erdani Kreft, M.; et al. Characterisation of plasmalemmal shedding of vesicles induced by the cholesterol/sphingomyelin binding protein, ostreolysin A-mCherry. Biochim. Biophys. Acta 2016, 1858, 2882–2893. [Google Scholar] [CrossRef]
- Rebolj, K.; Batista, U.; Sepčić, K.; Cestnik, V.; Maček, P.; Frangež, R. Ostreolysin affects rat aorta ring tension and endothelial cell viability in vitro. Toxicon 2007, 49, 1211–1213. [Google Scholar] [CrossRef]
- Glavan, G.; Kos, M.; Božič, J.; Drobne, D.; Sabotič, J.; Jemec, A.K. Different response of acetylcholinesterases in salt- and detergent-soluble fractions of honeybee haemolymph, head and thorax after exposure to diazinon. Comp. Biochem. Phys. C 2018, 205, 8–14. [Google Scholar] [CrossRef]
- Belmonte, G.; Pederzolli, C.; Maček, P.; Menestrina, G. Pore formation by the sea anemone cytolysin equinatoxin II in red blood cells and model lipid membranes. J. Membr. Biol. 1993, 131, 11–22. [Google Scholar] [CrossRef]
- OECD. Test No. 245: Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding); OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD Publishing: Paris, France, 2017. [Google Scholar]

| EryA/PlyB | Body Mass | Relative Organ Mass (% Body Mass) | |||
|---|---|---|---|---|---|
| (mg/kg) | (g) | Liver | Lung | Kidney | Heart |
| 0 | 23.56 ± 0.41 | 4.33 ± 0.16 | 0.82 ± 0.25 | 0.67 ± 0.06 | 0.58 ± 0.05 |
| 0.5 | 24.40 ± 0.52 | 4.07 ± 0.05 | 0.83 ± 0.24 | 0.71 ± 0.02 | 0.57 ± 0.07 |
| 1.0 | 24.46 ± 1.53 | 4.25 ± 0.23 | 0.87 ± 0.14 | 0.67 ± 0.03 | 0.63 ± 0.13 |
| 3.0 | 24.50 ± 0.54 | 4.15 ± 0.15 | 0.83 ± 0.17 | 0.69 ± 0.02 | 0.58 ± 0.05 |
| p value | 0.304 | 0.097 | 0.977 | 0.245 | 0.720 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panevska, A.; Glavan, G.; Jemec Kokalj, A.; Kukuljan, V.; Trobec, T.; Žužek, M.C.; Vrecl, M.; Drobne, D.; Frangež, R.; Sepčić, K. Effects of Bioinsecticidal Aegerolysin-Based Cytolytic Complexes on Non-Target Organisms. Toxins 2021, 13, 457. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070457
Panevska A, Glavan G, Jemec Kokalj A, Kukuljan V, Trobec T, Žužek MC, Vrecl M, Drobne D, Frangež R, Sepčić K. Effects of Bioinsecticidal Aegerolysin-Based Cytolytic Complexes on Non-Target Organisms. Toxins. 2021; 13(7):457. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070457
Chicago/Turabian StylePanevska, Anastasija, Gordana Glavan, Anita Jemec Kokalj, Veronika Kukuljan, Tomaž Trobec, Monika Cecilija Žužek, Milka Vrecl, Damjana Drobne, Robert Frangež, and Kristina Sepčić. 2021. "Effects of Bioinsecticidal Aegerolysin-Based Cytolytic Complexes on Non-Target Organisms" Toxins 13, no. 7: 457. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070457