Effect of Ambient pH on Growth, Pathogenicity, and Patulin Production of Penicillium expansum
Abstract
:1. Introduction
2. Results
2.1. Effect of Ambient pH on P. expansum Growth In Vitro
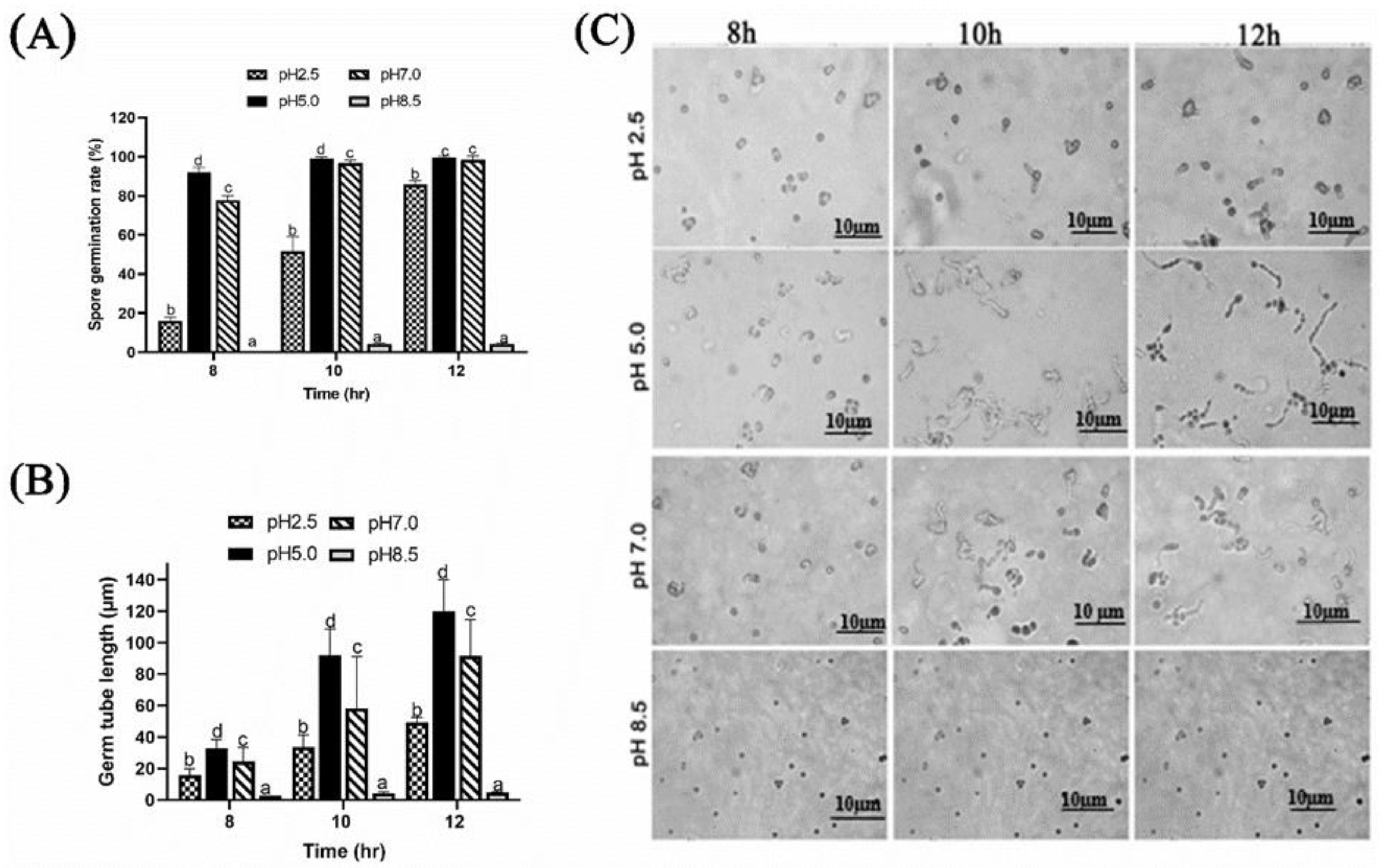
2.1.1. Effect of Different Ambient pH on Spore Germination and Germ Tube Length of P. expansum
2.1.2. Effect of Different Ambient pH on Colony Diameter and Colony Edges of P. expansum
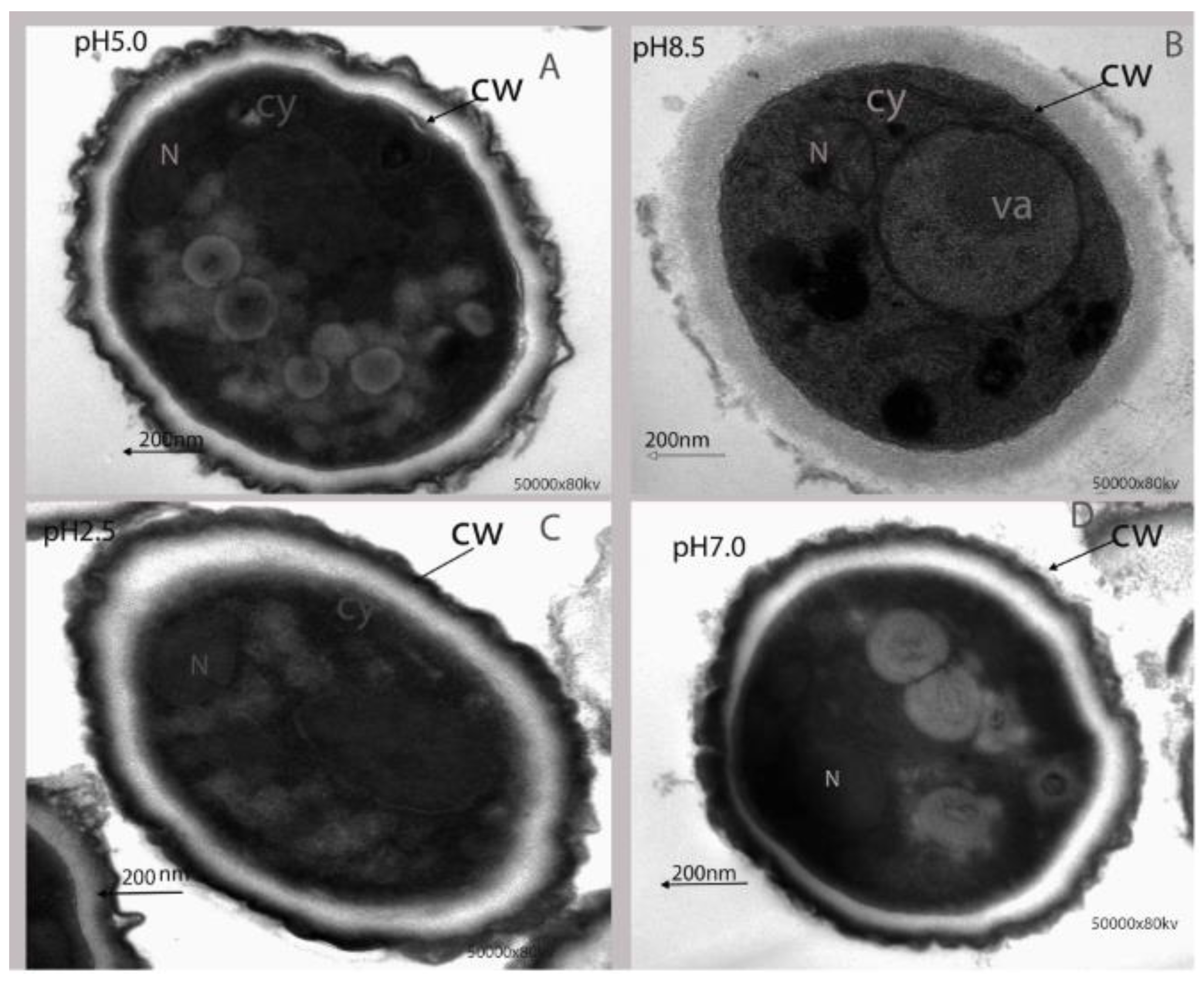
2.1.3. Effect of Ambient pH on Ultrastructural Alteration of P. expansum
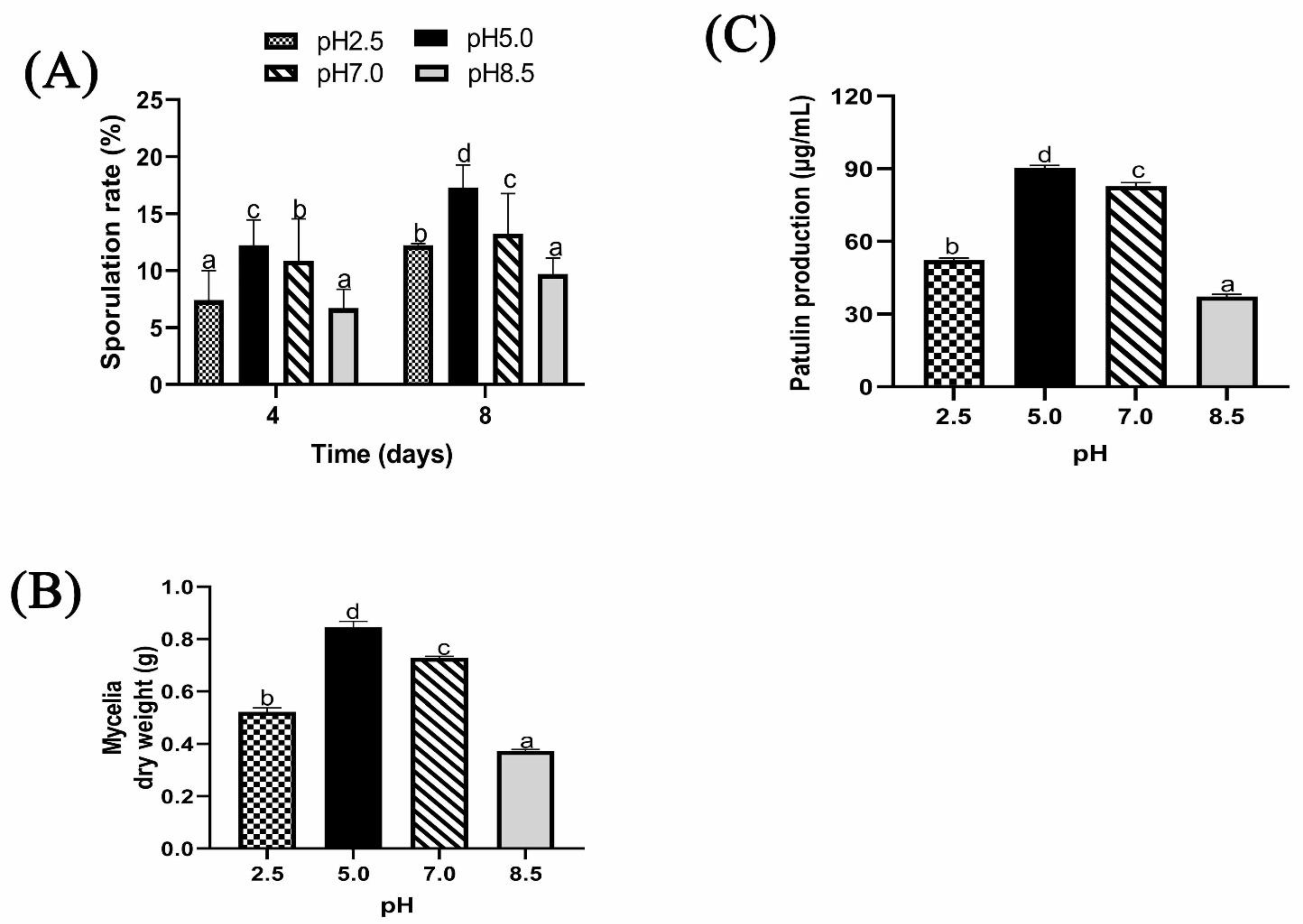
2.1.4. Effect of Different Ambient pH on Sporulation, Biomass, and Patulin Production of P. expansum
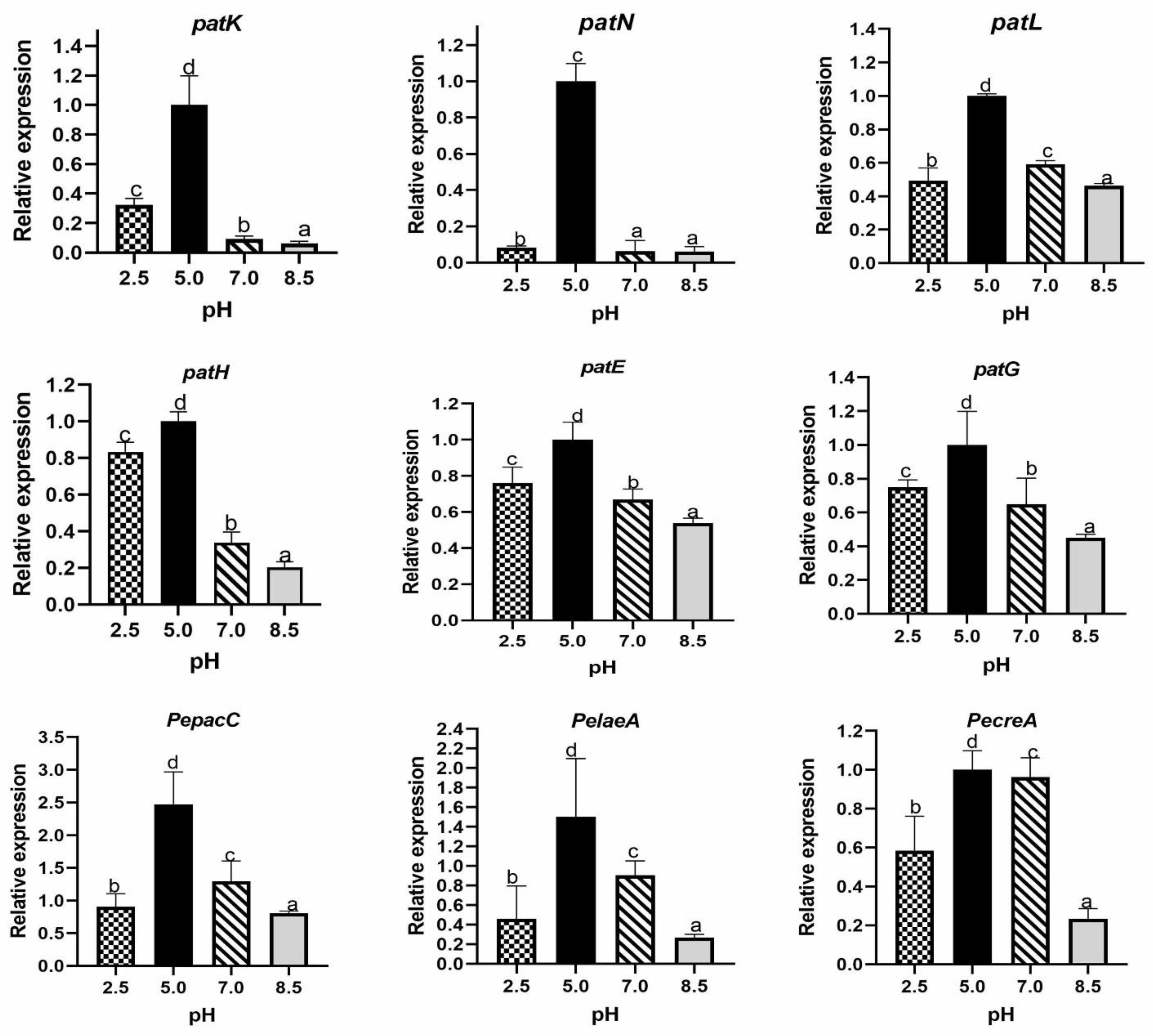
2.1.5. Effect of Ambient pH on Relative Expression of Genes Encoding Transcription Factors and Proteins Involved in Patulin Biosynthesis
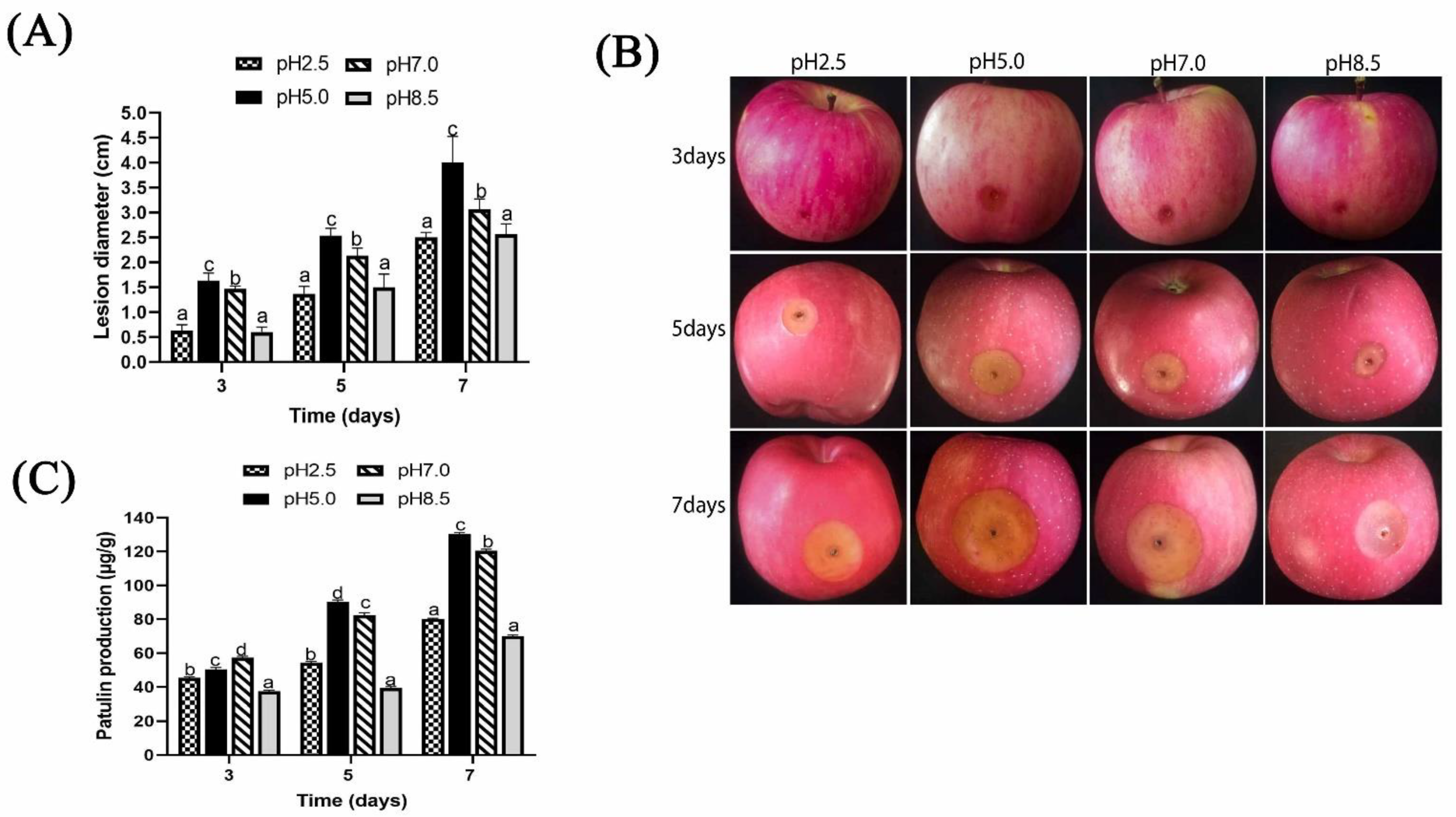
2.2. Effect of Ambient pH on Pathogenicity Induced by P. expansum and Patulin Production In Vivo
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Apple Fruits
5.2. Fungal Strain and Basal Medium Preparation
5.3. Studies on the Effect of Ambient pH on Growth Factors of P. expansum In Vitro
5.3.1. Studies on the Effect of Ambient pH on Spore Germination and Germ Tube Elongation of P. expansum
5.3.2. Studies on the Effect of Ambient pH on Colony Diameter and Colony Edges of P. expansum
5.3.3. Studies on the Effect of Ambient pH on Ultrastructural Alteration of P. expansum
5.3.4. Studies on the Effect of Ambient pH on Sporulation, Mycelia Biomass and Patulin Production
5.3.5. Studies on the Effect of Ambient pH on Expression of Genes Involved in Patulin Biosynthesis In Vitro
Primer Design and Total RNA Extraction
RT-qPCR and Relative mRNA Expression Analysis
5.3.6. Studies on the Effect of Ambient pH on Pathogenicity and Patulin Accumulation in Apple Fruits Inoculated with P. expansum In Vivo
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tannous, J.; Barda, O.; Luciano-Rosario, D.; Prusky, D.B.; Sionov, E.; Keller, N.P. New Insight Into Pathogenicity and Secondary Metabolism of the Plant Pathogen Penicillium expansum Through Deletion of the Epigenetic Reader SntB. Front. Microbiol. 2020, 11, 610. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.; Chen, Y.; Li, B.; Qin, G.; Tian, S. Mechanism of Penicillium expansum in Response to Exogenous Nitric Oxide Based on Proteomics Analysis. J. Proteom. 2014, 103, 47–56. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential Regulation by Ambient PH of Putative Virulence Factor Secretion by the Phytopathogenic Fungus Botrytis Cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef]
- Zong, Y.; Li, B.; Tian, S. Effects of Carbon, Nitrogen and Ambient PH on Patulin Production and Related Gene Expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef]
- Damoglou, A.P.; Campbell, D.S. The Effect of PH on the Production of Patulin in Apple Juice. Lett. Appl. Microbiol. 1986, 2, 9–11. [Google Scholar] [CrossRef]
- Hadas, Y.; Goldberg, I.; Pines, O.; Prusky, D. Involvement of Gluconic Acid and Glucose Oxidase in the Pathogenicity of Penicillium expansum in Apples. Phytopathology 2007, 97, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship Between Host Acidification and Virulence of Penicillium Spp. on Apple and Citrus Fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, W.; Chun, Y.W.; Yun, B.W.; Jian, J.X.; Zheng, L.; Jing, J.L.; Li, G.G.; Chang, K.S. Effect of Different pH Values on Growth and Sporulation of Estye Vermicola. Afr. J. Microbiol. Res. 2013, 7, 3217–3221. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, B.; Xu, X.; Zhang, Z.; Tian, S. The PH-Responsive PacC Transcription Factor Plays Pivotal Roles in Virulence and Patulin Biosynthesis in Penicillium expansum: Functional Characterization of PePacC. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Arst, H.N. Regulation of Gene Expression by Ambient pH in Filamentous Fungi and Yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 426–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñalva, M.A.; Tilburn, J.; Bignell, E.; Arst, H.N. Ambient pH Gene Regulation in Fungi: Making Connections. Trends Microbiol. 2008, 16, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zong, Y.; Du, Z.; Chen, Y.; Zhang, Z.; Qin, G.; Zhao, W.; Tian, S. Genomic Characterization Reveals Insights Into Patulin Biosynthesis and Pathogenicity in Penicillium Species. Mol. Plant-Microbe Interact. 2015, 28, 635–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannous, J.; El Khoury, R.; Snini, S.P.; Lippi, Y.; El Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, Physical Organization and Kinetic Expression of the Patulin Biosynthetic Gene Cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.-R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, Transcriptome, and Functional Analyses of Penicillium expansum Provide New Insights into Secondary Metabolism and Pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, H.; Marín, S.; Ramos, A.J.; Sanchis, V. Influence of Post-Harvest Technologies Applied during Cold Storage of Apples in Penicillium expansum Growth and Patulin Accumulation: A Review. Food Control 2010, 21, 953–962. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Luo, J.; Ye, B.; Zhou, Y.; Zhou, L.; Lai, T. Identification of Differentially Expressed Genes Involved in Spore Germination of Penicillium expansum by Comparative Transcriptome and Proteome Approaches. Microbiologyopen 2018, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Z. Proteomic Responses of Fruits to Environmental Stresses. Front. Plant Sci. 2013, 3, 311. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lai, T.; Qin, G.; Tian, S. Ambient pH Stress Inhibits Spore Germination of Penicillium expansum by Impairing Protein Synthesis and Folding: A Proteomic-Based Study. J. Proteome Res. 2010, 9, 298–307. [Google Scholar] [CrossRef]
- Tannous, J.; Atoui, A.; El Khoury, A.; Francis, Z.; Oswald, I.P.; Puel, O.; Lteif, R. A Study on the Physicochemical Parameters forPenicillium expansum Growth and Patulin Production: Effect of Temperature, pH, and Water Activity. Food Sci. Nutr. 2016, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Morales, H.; Barros, G.; Marín, S.; Chulze, S.; Ramos, A.J.; Sanchis, V. Effects of Apple and Pear Varieties and pH on Patulin Accumulation by Penicillium expansum. J. Sci. Food Agric. 2008, 88, 2738–2743. [Google Scholar] [CrossRef]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH Regulation of Sterigmatocystin and Aflatoxin Biosynthesis in Aspergillus Spp. Phytopathology 1997, 87, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-Mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Bu’Lock, J.D. Intermediary Metabolism and Antibiotic Synthesis. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1961; Volume 3, pp. 293–342. ISBN 978-0-12-002603-6. [Google Scholar]
- Sekiguchi, J.; Gaucher, G.M. Conidiogenesis and Secondary Metabolism in Penicillium Urticae. Appl. Environ. Microbiol. 1977, 33, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y.; Zhang, Z.; Xu, X.; Wang, X.; Long, M.; Tian, S. Dissection of Patulin Biosynthesis, Spatial Control and Regulation Mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin Is a Cultivar-Dependent Aggressiveness Factor Favouring the Colonization of Apples by Enicillium Expansum: Patulin is an Aggressiveness Factor. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef]
- White, S.; O’Callaghan, J.; Dobson, A.D.W. Cloning and Molecular Characterization of Penicillium expansum Genes Upregulated under Conditions Permissive for Patulin Biosynthesis. FEMS Microbiol. Lett. 2006, 255, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñalva, M.A.; Arst, J.H.N. Recent Advances in the Characterization of Ambient pH Regulation of Gene Expression in Filamentous Fungi and Yeasts. Annu. Rev. Microbiol. 2004, 58, 425–451. [Google Scholar] [CrossRef]
- Merhej, J.; Richard-Forget, F.; Barreau, C. The PH Regulatory Factor Pac1 Regulates Tri Gene Expression and Trichothecene Production in Fusarium Graminearum. Fungal Genet. Biol. 2011, 48, 275–284. [Google Scholar] [CrossRef]
- Mingot, J.M.; Espeso, E.A.; Díez, E.; Peñalva, M. Ambient pH Signaling Regulates Nuclear Localization of the Aspergillus nidulans PacC Transcription Factor. Mol. Cell. Biol. 2001, 21, 1688–1699. [Google Scholar] [CrossRef] [Green Version]
- Suárez, T.; Peñalva, M.A. Characterization of a Penicillium chrysogenum Gene Encoding a PacC Transcription Factor and Its Binding Sites in the Divergent PcbAB–PcbC Promoter of the Penicillin Biosynthetic Cluster. Mol. Microbiol. 1996, 20, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Tannous, J.; Sionov, E.; Keller, N.; Prusky, D. Apple Intrinsic Factors Modulating the Global Regulator, LaeA, the Patulin Gene Cluster and Patulin Accumulation During Fruit Colonization by Penicillium expansum. Front. Plant Sci. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barad, S.; Sionov, E.; Prusky, D. Role of Patulin in Post-Harvest Diseases. Fungal Biol. Rev. 2016, 30, 24–32. [Google Scholar] [CrossRef]
- Dowzer, C.E.A.; Kelly, J.M. Cloning of the CreA Gene from Aspergillus nidulans: A Gene Involved in Carbon Catabolite Repression. Curr. Genet. 1989, 15, 457–459. [Google Scholar] [CrossRef]
- Tannous, J.; Kumar, D.; Sela, N.; Sionov, E.; Prusky, D.; Keller, N.P. Fungal Attack and Host Defence Pathways Unveiled in Near-Avirulent Interactions of Penicillium expansum CreA Mutants on Apples: Fungal Attack and Host Defense Pathways. Mol. Plant Pathol. 2018, 19, 2635–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the Role of Patulin on Pathogenicity and Virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef]
- Qin, G.; Tian, S.; Chan, Z.; Li, B. Crucial Role of Antioxidant Proteins and Hydrolytic Enzymes in Pathogenicity of Penicillium expansum. Mol. Cell. Proteom. 2007, 6, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, S.; Long, M.; Gilbert, J.; Felgueiras, I. Liquid chromatographic method for determination of patulin in clear and cloudy apple juices and apple puree: Collaborative study. J. AOAC Int. 2000, 83, 1387–1394. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xue, H.; Bi, Y.; Wei, J.; Tang, Y.; Zhao, Y.; Wang, Y. New Method for the Simultaneous Analysis of Types A and B Trichothecenes by Ultrahigh-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry in Potato Tubers Inoculated with Fusarium sulphureum. J. Agric. Food Chem. 2013, 61, 9333–9338. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences (5’-3’) |
|---|---|
| PepatE | F: CATTCTCATCGGGCCTGAGT |
| R: TCGAAGCTCTTCCGGACATG | |
| PepatN | F: CGTTCGATGTCGCTAGCAAA |
| R: GGCGATAATCACGTCAATTCG | |
| PepatL | F: GCAGGAGATCCGTTTCAGACA |
| R: CCACTGACCGACGGTTACAAC | |
| PepatK | F: GACGCTGGGCTACTGGATTG |
| R: TCGTGCGTGAGGCCAGTAT | |
| PepatG | F: CGGCCGTCTTGAAGGAAAT |
| R: CTTGCCGTAGCGGGTGAATA | |
| PepatH | F: CATTTATCGGCGGTGTTCTGA |
| R: GATCAACGCTTGCACGATAGC | |
| PecreaA | F: CGATACTTCGCCCGACTCA |
| R: TGAGAGGCGGTAGCAAGCTAA | |
| PelaeA | F: CCCGAGAAATACCCGAATCA |
| R: TCACACGGAAGCGGGTAGAT | |
| PepacC | F: TGAGGCTGGTACTGCCGAAT |
| R: CAACTCCTTCCATGGCATCA | |
| β-tubulin | F: CTCCAGCTCGAGCGTATGAA |
| R: GGCTCCAAATCGACGAGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimdjio, C.K.; Xue, H.; Bi, Y.; Nan, M.; Li, L.; Zhang, R.; Liu, Q.; Pu, L. Effect of Ambient pH on Growth, Pathogenicity, and Patulin Production of Penicillium expansum. Toxins 2021, 13, 550. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080550
Jimdjio CK, Xue H, Bi Y, Nan M, Li L, Zhang R, Liu Q, Pu L. Effect of Ambient pH on Growth, Pathogenicity, and Patulin Production of Penicillium expansum. Toxins. 2021; 13(8):550. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080550
Chicago/Turabian StyleJimdjio, Carelle Kouasseu, Huali Xue, Yang Bi, Mina Nan, Lan Li, Rui Zhang, Qili Liu, and Lumei Pu. 2021. "Effect of Ambient pH on Growth, Pathogenicity, and Patulin Production of Penicillium expansum" Toxins 13, no. 8: 550. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080550





