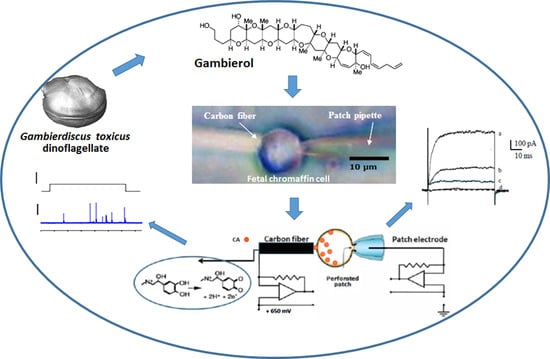
Gambierol Blocks a K+ Current Fraction without Affecting Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of Gambierol on Outward K+ Current in Rat Fetal Adrenomedullary Chromaffin Cells
2.2. Effect of Gambierol on Cathecholamine Release
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Toxins
5.2. Animals
5.3. Rat Fetal Adrenomedullary Chromaffin Cell Cultures
5.4. Whole-Cell Voltage- and Current-Clamp Recordings
5.5. Amperometric Recordings from Single Cells
5.6. Statistics and Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Shmukler, Y.B.; Nikishin, D.A. Ladder-Shaped Ion Channel Ligands: Current State of Knowledge. Mar. Drugs 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Fuwa, H. Convergent strategies for the total synthesis of polycyclic ether marine metabolites. Nat. Prod. Rep. 2008, 25, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Frederick, M.O.; Aversa, R.J. The continuing saga of the marine polyether biotoxins. Angew. Chem. Int. Ed. Engl. 2008, 47, 7182–7225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y. Development of New Synthetic Methods Using Oxiranyl Anions and Application in the Syntheses of Polycyclic Ether Marine Natural Products. Chem. Pharm. Bull. 2019, 67, 1–17. [Google Scholar] [CrossRef]
- Wan, X.; Yao, G.; Liu, Y.; Chen, J.; Jiang, H. Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins. Mar. Drugs 2019, 17, 594. [Google Scholar] [CrossRef] [Green Version]
- Fuwa, H.; Sasaki, M.; Satake, M.; Tachibana, K. Total synthesis of gambierol. Org. Lett. 2002, 4, 2981–2984. [Google Scholar] [CrossRef]
- Johnson, H.W.; Majumder, U.; Rainier, J.D. Total synthesis of gambierol: Subunit coupling and completion. Chemistry 2006, 12, 1747–1753. [Google Scholar] [CrossRef]
- Fuwa, H.; Sasaki, M. Recent advances in the synthesis of marine polycyclic ether natural products. Curr. Opin. Drug Discov. Devel. 2007, 10, 784–806. [Google Scholar]
- Alonso, E.; Fuwa, H.; Vale, C.; Suga, Y.; Goto, T.; Konno, Y.; Sasaki, M.; LaFerla, F.M.; Vieytes, M.R.; Giménez-Llort, L.; et al. Design and synthesis of skeletal analogues of gambierol: Attenuation of amyloid-β and tau pathology with voltage-gated potassium channel and N-methyl-D-aspartate receptor implications. J. Am. Chem. Soc. 2012, 134, 7467–7479. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Morohashi, A.; Satake, M.; Yasumoto, T. The absolute configuration of gambierol, a toxic marine polyether from the dinoflagellate, Gambierdiscus toxicus. Tetrahedron Lett. 1999, 40, 97–100. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2021, 102, 101873. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Zimmerman, K.; Lewis, R.J. Ciguatera toxins: Pharmacology, toxicology, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; Chapter 32; pp. 925–950. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera-causing dinoflagellates in the genera Gambierdiscus and Fukuyoa: Distribution, ecophysiology and toxicology. In Dinoflagellates: Morphology, Life History and Ecological Significance; Subba Rao, D.V., Ed.; Nova Science: New York, NY, USA, 2020; pp. 405–457. ISBN 978-1-53617-888-3. [Google Scholar]
- Ghiaroni, V.; Sasaki, M.; Fuwa, H.; Rossini, G.P.; Scalera, G.; Yasumoto, T.; Pietra, P.; Bigiani, A. Inhibition of voltage-gated potassium currents by gambierol in mouse taste cells. Toxicol. Sci. 2005, 85, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, S.; Ouanounou, G.; Girard, E.; Sasaki, M.; Fuwa, H.; Louzao, M.C.; Botana, L.M.; Benoit, E.; Molgó, J. The marine polyether gambierol enhances muscle contraction and blocks a transient K+ current in skeletal muscle cells. Toxicon 2010, 56, 785–791. [Google Scholar] [CrossRef]
- Pérez, S.; Vale, C.; Alonso, E.; Fuwa, H.; Sasaki, M.; Konno, Y.; Goto, T.; Suga, Y.; Vieytes, M.R.; Botana, L.M. Effect of gambierol and its tetracyclic and heptacyclic analogues in cultured cerebellar neurons: A structure-activity relationships study. Chem. Res. Toxicol 2012, 25, 1929–1937. [Google Scholar] [CrossRef]
- Cuypers, E.; Abdel-Mottaleb, Y.; Kopljar, I.; Rainier, J.D.; Raes, A.L.; Snyders, D.J.; Tytgat, J. Gambierol, a toxin produced by the dinoflagellate Gambierdiscus toxicus, is a potent blocker of voltage-gated potassium channels. Toxicon 2008, 51, 974–983. [Google Scholar] [CrossRef] [Green Version]
- Konoki, K.; Suga, Y.; Fuwa, H.; Yotsu-Yamashita, M.; Sasaki, M. Evaluation of gambierol and its analogs for their inhibition of human Kv1.2 and cytotoxicity. Bioorg. Med. Chem. Lett. 2015, 25, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.A.; Vale, C.; Martín, V.; Fuwa, H.; Sasaki, M.; Botana, L.M. Potassium currents inhibition by gambierol analogs prevents human T lymphocyte activation. Arch. Toxicol. 2015, 89, 1119–1134. [Google Scholar] [CrossRef]
- Kopljar, I.; Labro, A.J.; de Block, T.; Rainier, J.D.; Tytgat, J.; Snyders, D.J. The ladder-shaped polyether toxin gambierol anchors the gating machinery of Kv3.1 channels in the resting state. J. Gen. Physiol. 2013, 141, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molgó, J.; Schlumberger, S.; Sasaki, M.; Fuwa, H.; Louzao, M.C.; Botana, L.M.; Servent, D.; Benoit, E. Gambierol Potently Increases Evoked Quantal Transmitter Release and Reverses Pre- and Post-Synaptic Blockade at Vertebrate Neuromuscular Junctions. Neuroscience 2020, 439, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Molgó, J.; Schlumberger, S.; Sasaki, M.; Fuwa, H.; Louzao, M.C.; Botana, L.M.; Servent, D.; Benoit, E. Gambierol enhances evoked quantal transmitter release and blocks a potassium current in motor nerve terminals of the mouse neuromuscular junction. In Harmful Algae 2018–From Ecosystems to Socio Ecosystems. Proceedings of the 18th International Conference on Harmful Algae; Hess, P., Ed.; International Society for the Study of Harmful Algae: Nantes, France, 2020; pp. 145–148. [Google Scholar]
- Kidokoro, Y.; Ritchie, A.K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J. Physiol. 1980, 307, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenwick, E.M.; Marty, A.; Neher, E. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 1982, 331, 599–635. [Google Scholar] [CrossRef]
- Martinez-Espinosa, P.L.; Neely, A.; Ding, J.; Lingle, C.J. Fast inactivation of Nav current in rat adrenal chromaffin cells involves two independent inactivation pathways. J. Gen. Physiol. 2021, 153, e202012784. [Google Scholar] [CrossRef]
- Lingle, C.J.; Martinez-Espinosa, P.L.; Guarina, L.; Carbone, E. Roles of Na+, Ca2+, and K+ channels in the generation of repetitive firing and rhythmic bursting in adrenal chromaffin cells. Pflügers Arch. 2018, 470, 39–52. [Google Scholar] [CrossRef]
- Bournaud, R.; Hidalgo, J.; Yu, H.; Jaimovich, E.; Shimahara, T. Low threshold T-type calcium current in rat embryonic chromaffin cells. J. Physiol. 2001, 537, 35–44. [Google Scholar] [CrossRef]
- Favreau, P.; Gilles, N.; Lamthanh, H.; Bournaud, R.; Shimahara, T.; Bouet, F.; Laboute, P.; Letourneux, Y.; Ménez, A.; Molgó, J.; et al. A new omega-conotoxin that targets N-type voltage-sensitive calcium channels with unusual specificity. Biochemistry 2001, 40, 14567–14575. [Google Scholar] [CrossRef]
- García, A.G.; García-De-Diego, A.M.; Gandía, L.; Borges, R.; García-Sancho, J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol. Rev. 2006, 86, 1093–1131. [Google Scholar] [CrossRef] [Green Version]
- Padín, J.F.; Fernández-Morales, J.C.; de Diego, A.M.; García, A.G. Calcium Channel Subtypes and Exocytosis in Chromaffin Cells at Early Life. Curr. Mol. Pharmacol. 2015, 8, 81–86. [Google Scholar] [CrossRef]
- Nurse, C.A.; Salman, S.; Scott, A.L. Hypoxia-regulated catecholamine secretion in chromaffin cells. Cell Tissue Res. 2018, 372, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Seidler, F.J.; Slotkin, T.A. Adrenomedullary function in the neonatal rat: Responses to acute hypoxia. J. Physiol. 1985, 358, 1–16. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J. Adrenomedullary catecholamine release in the fetus and newborn: Secretory mechanisms and their role in stress and survival. J. Dev. Physiol. 1988, 10, 1–16. [Google Scholar] [PubMed]
- Bournaud, R.; Hidalgo, J.; Yu, H.; Girard, E.; Shimahara, T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflügers Arch. 2007, 454, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Nurse, C.A. Anoxia differentially modulates multiple K+ currents and depolarizes neonatal rat adrenal chromaffin cells. J. Physiol. 1998, 512, 421–434. [Google Scholar] [CrossRef]
- Artalejo, A.R.; García, A.G.; Neher, E. Small-conductance Ca2+-activated K+ channels in bovine chromaffin cells. Pflug. Arch. 1993, 423, 97–103. [Google Scholar] [CrossRef]
- Candia, S.; Garcia, M.L.; Latorre, R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys. J. 1992, 63, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Salman, S.; Buttigieg, J.; Zhang, M.; Nurse, C.A. Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity. J. Physiol. 2013, 591, 515–529. [Google Scholar] [CrossRef]
- Zhou, Z.; Misler, S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J. Biol. Chem. 1995, 270, 3498–3505. [Google Scholar] [CrossRef] [Green Version]
- Ghiaroni, V.; Fuwa, H.; Inoue, M.; Sasaki, M.; Miyazaki, K.; Hirama, M.; Yasumoto, T.; Rossini, G.P.; Scalera, G.; Bigiani, A. Effect of ciguatoxin 3C on voltage-gated Na+ and K+ currents in mouse taste cells. Chem. Senses 2006, 31, 673–680. [Google Scholar] [CrossRef]
- Hsu, H.T.; Lo, Y.C.; Huang, Y.M.; Tseng, Y.T.; Wu, S.N. Important modifications by sugammadex, a modified γ-cyclodextrin, of ion currents in differentiated NSC-34 neuronal cells. BMC Neurosci. 2017, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopljar, I.; Labro, A.J.; Cuypers, E.; Johnson, H.W.; Rainier, J.D.; Tytgat, J.; Snyders, D.J. A polyether biotoxin binding site on the lipid-exposed face of the pore domain of Kv channels revealed by the marine toxin gambierol. Proc. Natl. Acad. Sci. USA 2009, 106, 9896–9901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, J.; Liberona, J.L.; Molgó, J.; Jaimovich, E. Pacific ciguatoxin-1b effect over Na+ and K+ currents, inositol 1,4,5-triphosphate content and intracellular Ca2+ signals in cultured rat myotubes. Br. J. Pharmacol. 2002, 137, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birinyi-Strachan, L.C.; Gunning, S.J.; Lewis, R.J.; Nicholson, G.M. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol. Appl. Pharmacol. 2005, 204, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, S.; Mattei, C.; Molgó, J.; Benoit, E. Dual action of a dinoflagellate-derived precursor of Pacific ciguatoxins (P-CTX-4B) on voltage-dependent K+ and Na+ channels of single myelinated axons. Toxicon 2010, 56, 768–775. [Google Scholar] [CrossRef]
- Kopljar, I.; Grottesi, A.; de Block, T.; Rainier, J.D.; Tytgat, J.; Labro, A.J.; Snyders, D.J. Voltage-sensor conformation shapes the intra-membrane drug binding site that determines gambierol affinity in Kv channels. Neuropharmacology 2016, 107, 160–167. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Buttigieg, J.; Brown, S.; Holloway, A.C.; Nurse, C.A. Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: Role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha. J. Neurosci. 2009, 29, 7137–7147. [Google Scholar] [CrossRef] [Green Version]
- Wightman, R.M.; Jankowski, J.A.; Kennedy, R.T.; Kawagoe, K.T.; Schroeder, T.J.; Leszczyszyn, D.J.; Near, J.A.; Diliberto, E.J., Jr.; Viveros, O.H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10754–10758. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benoit, E.; Schlumberger, S.; Molgó, J.; Sasaki, M.; Fuwa, H.; Bournaud, R. Gambierol Blocks a K+ Current Fraction without Affecting Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells. Toxins 2022, 14, 254. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14040254
Benoit E, Schlumberger S, Molgó J, Sasaki M, Fuwa H, Bournaud R. Gambierol Blocks a K+ Current Fraction without Affecting Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells. Toxins. 2022; 14(4):254. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14040254
Chicago/Turabian StyleBenoit, Evelyne, Sébastien Schlumberger, Jordi Molgó, Makoto Sasaki, Haruhiko Fuwa, and Roland Bournaud. 2022. "Gambierol Blocks a K+ Current Fraction without Affecting Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells" Toxins 14, no. 4: 254. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14040254