Evaluation of the Adsorption Efficacy of Bentonite on Aflatoxin M1 Levels in Contaminated Milk
Abstract
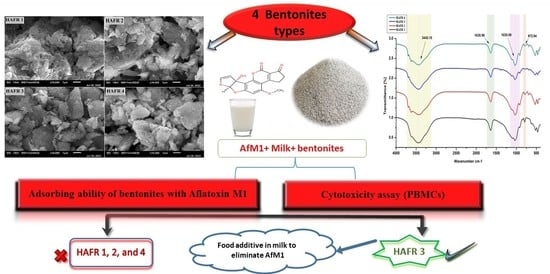
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic and Microscopic Characterization of Bentonites
2.2. Concentration and Frequency of Detection of Aflatoxins M1 in Raw Milk Samples Using HPLC
2.2.1. Adsorption Capability of AFM1 in PBS Solution by Various Bentonite HAFR Types
2.3. Effect of Bentonites on Qualitative Characteristics of the Milk Samples
2.4. Cytotoxicity Evaluation of Bentonites
2.5. X-ray Diffraction of Different Types of Bentonites
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sampling
Sample Preparation
4.3. Cleanup/Purification and HPLC Conditions
4.4. Adsorption of AFM1 in Phosphate Buffer Saline (PBS) by Different Types of Bentonite Samples
4.5. Adsorption of AFM1 in Milk Samples by Bentonite (HAFR 1 and HAFR 3)
4.6. Qualitative Properties of the Tested Milk
4.7. Cytotoxicity Assessment of Bentonites
4.8. Bentonite Characterization
4.8.1. X-ray Diffraction (XRD) Analysis
4.8.2. Fourier-Transform Infrared (FTIR)
4.8.3. Scanning Electron Microscopy (SEM)
4.8.4. Transmission Electron Microscope (TEM)
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Aflatoxins. In Food Safety Digest; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Petrova, P.; Arsov, A.; Tsvetanova, F.; Parvanova-Mancheva, T.; Vasileva, E.; Tsigoriyna, L.; Petrov, K. The Complex Role of Lactic Acid Bacteria in Food Detoxification. Nutrients 2022, 14, 2038. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Campagnollo, F.B.; Ganev, K.C.; Khaneghah, A.M.; Portela, J.B.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: A review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and other biogenic amines in food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- Patyal, A.; Gill, J.P.S.; Bedi, J.S.; Aulakh, R.S. Potential risk factors associated with the occurrence of aflatoxin M1 in raw milk produced under different farm conditions. J. Environ. Sci. Health. Part. B 2020, 55, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Flint, S.; Palmer, J. Control of aflatoxin M1 in milk by novel methods: A review. Food Chem. 2020, 311, 125984. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Amer, A.; El-Nogoumy, B.; Ibrahim, M.; Hassan, S.; Siddiqui, S.A.; EL-Gazzar, A.M.; Khalifa, E.; Omar, S.A.; Abou-Alella, S.A.; et al. Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate. Toxins 2023, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.S. Aflatoxin M1 levels in raw milk, pasteurised milk and infant formula. Ital. J. Food Saf. 2016, 5, 5788. [Google Scholar] [PubMed] [Green Version]
- Huff, W.D. K-bentonites: A review. Am. Mineral. 2016, 101, 43–70. [Google Scholar] [CrossRef]
- Nones, J.; Nones, J.; Poli, A.; Trentin, A.G.; Riella, H.G.; Kuhnen, N.C. Organophilic treatments of bentonite increase the adsorption of aflatoxin B1 and protect stem cells against cellular damage. Colloids Surf. B. 2016, 145, 555–561. [Google Scholar] [CrossRef]
- Zhou, H. Mixture of palygorskite and montmorillonite (Paly-Mont) and its adsorptive application for mycotoxins. Appl. Clay. Sci. 2016, 131, 140–143. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Giovati, L.; Magliani, W.; Ciociola, T.; Santinoli, C.; Conti, S.; Polonelli, L. AFM₁ in Milk: Physical, biological, and prophylactic methods to mitigate contamination. Toxins 2015, 7, 4330–4349. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, M.; Pletcher, D. Industrial Applications. In Electrochemistry in Research and Development; Kalvoda, R., Parsons, R., Eds.; Springer: Boston, MA, USA, 1985; pp. 261–281. [Google Scholar]
- Di Gregorio, M.C.; Neeff, D.V.D.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.D.P.; Albuquerque, R.D.; Azevedo, A.C.D.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Hamad, G.M.; El-Makarem, H.A.; Abd Elaziz, A.I.; Amer, A.A.; El-Nogoumy, B.A.; Abou-Alella, S.A. Adsorption efficiency of sodium & calcium bentonite for ochratoxin A in some Egyptian cheeses: An innovative fortification model, in vitro and in vivo experiments. World Mycotoxin J. 2022, 15, 285–300. [Google Scholar]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A. Assorted methods for decontamination of Aflatoxin M1 in milk using microbial adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Lawal, I.A.; Gbashi, S.; Njobeh, P.B. Decontamination of T-2 toxin in maize by modified montmorillonite clay. Toxins 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryuchkova, M.; Batasheva, S.; Akhatova, F.; Babaev, V.; Buzyurova, D.; Vikulina, A.; Volodkin, D.; Fakhrullin, R.; Rozhina, E. Pharmaceuticals removal by adsorption with montmorillonite nanoclay. Int. J. Mol. Sci. 2021, 22, 9670. [Google Scholar] [CrossRef] [PubMed]
- Agag, B.I. Prevention and control of mycotoxins in feeds. Assiut Univ. Bulletin. Environm. Res. 2003, 6, 149–166. [Google Scholar]
- Upadhaya, S.D.; Park, M.A.; Ha, J.K. Mycotoxins and their biotransformation in the rumen: A Review. Asian-Australas. J. Anim. Sci. 2010, 23, 1250–1260. [Google Scholar] [CrossRef]
- Nones, J.; Solhaug, A.; Eriksen, G.S.; Macuvele, D.L.P.; Poli, A.; Soares, C.; Trentin, A.G.; Riella, H.G.; Nones, J. Bentonite modified with zinc enhances aflatoxin B1 adsorption and increase survival of fibroblasts (3T3) and epithelial colorectal adenocarcinoma cells (Caco-2). J. Hazard. Mater. 2017, 337, 80–89. [Google Scholar] [CrossRef]
- Ikhtiyarova, G.A.; Özcan, A.S.; Gök, Ö.; Özcan, A. Characterization of natural- and organo-bentonite by XRD, SEM, FT-IR and thermal analysis techniques and its adsorption behaviour in aqueous solutions. Clay Miner. 2012, 47, 31–44. [Google Scholar] [CrossRef]
- Sani, N.; Abdulsalam, A.K.; Abdullahi, U.A. Extraction and quantification of silicon from silica sand obtained from zauma river, zamfara state, Nigeria. Eur. Sci. J. 2013, 99, 160–168. [Google Scholar]
- Deepracha, S.; Vibulyaseak, K.G.; Ogawa, M. Complexation of TiO2 with clays and clay minerals for hierarchically designed functional hybrids. Advanced Supramolecular Nanoarchitectonics; William Andrew Publishing: Norwich, NY, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–150. [Google Scholar] [CrossRef]
- Chihi, R.; Blidi, I.; Trabelsi-Ayadi, M.; Ayari, F. Elaboration and characterization of a low-cost porous ceramic support from natural Tunisian bentonite clay. C. R. Chim. 2019, 22, 188–197. [Google Scholar] [CrossRef]
- Pеtrović, Z.; Dugić, P.; Аlеksić, V.; Bеgić, S.; Sаdаdinović, Ј.; Мićić, V.; Kljajić, N. Composition, structure and textural characteristics of domestic acid activated bentonite. J. Ergon. Soc. Korea 2014, 1, 133–139. [Google Scholar] [CrossRef]
- Djomgoue, P.; Njopwouo, D. FT-IR spectroscopy applied for surface clays characterization. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.S. Postfermentation treatments and related topics—ScienceDirect. In Wine Science, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 573–723. [Google Scholar]
- Lubbers, S.; Charpentier, C.; Feuillat, M. Study of the binding of aroma compounds by bentonites in must, wine and model systems. Vitis 1996, 35, 59–62. [Google Scholar]
- Carraro, A.; De Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014, 88–89, 92–99. [Google Scholar] [CrossRef]
- De Matos, C.J.; Schabo, D.C.; do Nascimento, Y.M.; Tavares, J.F. Aflatoxin M1 in Brazilian goat milk and health risk assessment. J. Environ. Sci. Health Part B 2021, 56, 415–422. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Elhussein, A.M. Determination of aflatoxin M1 in dairy cattle milk in Khartoum State, Sudan. Food Control 2010, 21, 945–946. [Google Scholar] [CrossRef]
- Hussain, I.; Anwar, J.; Asi, M.R.; Munawar, M.A.; Kashif, M. Aflatoxin M1 contamination in milk from five dairy species in Pakistan. Food Control 2010, 21, 122–124. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Marth, E.H. Use of sulphite or bentonite to eliminate aflatoxin M1 from naturally contaminated raw whole milk. Z. Lebensm. Unters. Forsch. 1982, 174, 303–305. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Zartman, R.E.; Hudnall, W.H. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay. Sci. 2007, 36, 197–205. [Google Scholar] [CrossRef]
- Subhan, S.; Muhammad, Y.; Sahibzada, M.; Subhan, F.; Tong, Z. Studies on the selection of a catalyst–oxidant system for the energy-efficient desulfurization and denitrogenation of fuel oil at mild operating conditions. Energy Fuels 2019, 33, 8423–8439. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Kholif, A.E.-K.M. Mycotoxins in animal feeds and prevention strategies: A review. Asian J. Anim. Sci. 2008, 4, 113–131. [Google Scholar] [CrossRef]
- Asi, M.R.; Iqbal, S.Z.; Ariño, A.; Hussain, A. Effect of seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab, Pakistan. Food Control 2012, 25, 34–38. [Google Scholar] [CrossRef]
- Montaseri, H.; Arjmandtalab, S.; Dehghanzadeh, G.; Karami, S.; Oryan, A. Effect of production and storage of probiotic yogurt on aflatoxin M1 residue. J. Food Qual. Hazards Control 2014, 1, 7–14. [Google Scholar]
- Awasthi, V.; Bahman, S.; Thakur, L.K.; Singh, S.K.; Dua, A.; Ganguly, S. Contaminants in milk and impact of heating: An assessment study. Indian J. Public Health 2012, 56, 95–99. [Google Scholar]
- Naeimipour, F.; Aghajani, J.; Kojuri, S.A.; Ayoubi, S. Useful approaches for reducing aflatoxin M1 content in milk and dairy products. Biomed. Biotechnol. Res. J. 2018, 2, 94–99. [Google Scholar]
- Zhang, M.; Li, X.; Lu, Y.; Fang, X.; Chen, Q.; Xing, M.; He, J. Studying the genotoxic effects induced by two kinds of bentonite particles on human B lymphoblast cells in vitro. Mutat. Res. 2011, 720, 62–66. [Google Scholar] [CrossRef]
- Michel, C.; Herzog, S.; de Capitani, C.; Burkhardt-Holm, P.; Pietsch, C. Natural mineral particles are cytotoxic to rainbow trout gill epithelial cells in vitro. PLoS ONE 2014, 9, e100856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, A.; Peng, M.M.; Ganesh, M.; Jayaprakash, J.; Mohankumar, M.; Jang, H.T. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dahlous, K.A.; Abd-Elkader, O.H.; Fouda, M.M.G.; Al Othman, Z.; El-Faham, A. Eco-friendly method for silver nanoparticles immobilized decorated silica: Synthesis & characterization and preliminary antibacterial activity. J. Taiwan Inst. Chem. Eng. 2019, 95, 324–331. [Google Scholar]
- Murshed, S. Evaluation and Assessment of aflatoxin m1 in milk and milk products in yemen using high-performance liquid chromatography. J. Food Qual. 2020, 2020, 8839060. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S. Variation of aflatoxin M1 contamination in milk and milk products collected during winter and summer seasons. Food Control 2013, 34, 714–718. [Google Scholar] [CrossRef]
- Keskin, Y.; Baskaya, R.; Karsli, S.; Yurdun, T.; Ozyaral, O. Detection of aflatoxin M1 in human breast milk and raw cow’s milk in Istanbul, Turkey. J. Food Prot. 2009, 72, 885. [Google Scholar] [CrossRef]
- Jahanmard, E.; Keramat, J.; Nasirpour, A.; Emadi, R. Efficiency of calcined aluminum-magnesium layered double hydroxide for adsorption of aflatoxin m1 from solution and matrix of milk. J. Food Sci. 2021, 86, 5200–5212. [Google Scholar] [CrossRef]
- Bouffard, L.; Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development and Wellness; Guilford Press: New York, NY, USA, 2017. [Google Scholar]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef] [PubMed]




| Element (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | Cl | K | Ca | Ti | |
| HAFR (1) | 0.30 | 1.92 | 8.70 | 45.13 | 0 | 0.77 | 0.91 | 0 |
| HAFR (2) | 3.81 | 0.93 | 13.86 | 35.17 | 0.45 | 0.43 | 0.37 | 0.28 |
| HAFR (3) | 0.63 | 1.67 | 12.18 | 26.99 | 0 | 0.65 | 0.50 | 0.37 |
| HAFR (4) | 0.08 | 0.10 | 0.45 | 0.95 | 0.05 | 0.05 | 0.05 | 0.07 |
| All Raw Samples | Contaminated Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | N | Min–Max | Mean (±SD) | Median (Q1–Q3) | N (%) | N (%) > MRL | Min–Max | Mean (±SD) | Median (Q1–Q3) |
| Cow | 50 | nd–122.80 | 48.13 ± 34.58 | 51.05 (14.60–76.95) | 44 (88) | 26 (54) | 3.70–122.80 | 54.69 ± 31.55 | 54.90 (25.47–79.80) |
| Camel | 20 | nd–142.40 | 70.0 ± 50.35 | 76.4 (9.82–115.37) | 16 (80) | 14 (70) | 6.50–142.40 | 87.50 ± 39.73 | 82.59 (66.67–127.55) |
| Sheep | 15 | nd–320.3 | 145.93 ± 111.84 | 140.60 (26.40–230.70) | 13 (86.66) | 11 (73.33) | 9.6–320.3 | 168.38 ± 102.46 | 160.10 (81.40–260.55) |
| Goat | 15 | nd–490.30 | 221.28 ± 153.11 | 234.50 (98.40–360.20) | 14 (93.33) | 13 (86.66) | 5.60–490.30 | 237.08 ± 145.64 | 242.90 (118.05–367.67) |
| P1 = 0.001, P2 = 0.002 | |||||||||
| Samples | 0 h | 0.5 h | 1 h | 2 h | 3 h | 6 h | 12 h |
|---|---|---|---|---|---|---|---|
| PBS (−Ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS +(100 ng/L) AFM1 (+ve) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PBS + (1 g) bentonite (HAFR 1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (1 g) bentonite (HAFR 2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS +(1 g) bentonite (HAFR 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (1 g) bentonite (HAFR 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 1) | 100 | 83 | 72 | 63 | 55 | 52 | 50 |
| PBS +(100 ng/L) AFM1+ (1 g) bentonite (HAFR 1) | 100 | 65 | 44 | 33 | 10 | 4 | 1.5 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 2) | 100 | 82 | 71 | 62 | 55 | 53 | 51 |
| PBS + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 2) | 100 | 63 | 41 | 21 | 9 | 3 | 2 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 3) | 100 | 80 | 68 | 59 | 52 | 50 | 48 |
| PBS + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 3) | 100 | 60 | 38 | 18 | 5 | 1 | 0 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 4) | 100 | 88 | 76 | 62 | 55 | 53 | 52 |
| PBS + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 4) | 100 | 68 | 51 | 24 | 11 | 6 | 4 |
| Samples | 0 h | 0.5 h | 1 h | 2 h | 3 h | 6 h | 12 h |
|---|---|---|---|---|---|---|---|
| Adsorption (%) | |||||||
| PBS (−Ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS +(100 ng/L) AFM1 (+ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (1 g) bentonite (HAFR 1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (1 g) bentonite (HAFR 2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS +(1 g) bentonite (HAFR 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (1 g) bentonite (HAFR 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 1) | 0 | 17 | 28 | 37 | 45 | 48 | 50 |
| PBS +(100 ng/L) AFM1+ (1 g) bentonite (HAFR 1) | 0 | 35 | 56 | 77 | 90 | 96 | 98.5 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 2) | 0 | 18 | 29 | 38 | 45 | 47 | 49 |
| PBS + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 2) | 0 | 37 | 59 | 79 | 91 | 97 | 98 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 3) | 0 | 20 | 32 | 41 | 48 | 50 | 52 |
| PBS+ (100 ng/L) AFM1+ (1 g) bentonite (HAFR 3) | 0 | 40 | 62 | 82 | 95 | 99 | 100 |
| PBS + (100 ng/L) AFM1+ (0.5 g) bentonite (HAFR 4) | 0 | 12 | 24 | 38 | 45 | 47 | 48 |
| PBS + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 4) | 0 | 32 | 49 | 76 | 89 | 94 | 96 |
| Samples | 0 h | 0.5 h | 1 h | 2 h | 3 h | 6 h | 12 h |
|---|---|---|---|---|---|---|---|
| Milk (−Ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk +(100 ng/L) AFM1 (+ve) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Milk + (1 g) bentonite (HAFR 1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk + (1 g) bentonite (HAFR 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 1) | 100 | 67 | 46 | 35 | 13 | 7 | 5 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 3) | 100 | 63 | 40 | 20 | 7 | 5 | 1.5 |
| Samples | 0 h | 0.5 h | 1 h | 2 h | 3 h | 6 h | 12 h |
|---|---|---|---|---|---|---|---|
| Adsorption (%) | |||||||
| Milk (−Ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk +(100 ng/L) AFM1 (+ve) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk + (1 g) bentonite (HAFR 1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk + (1 g) bentonite (HAFR 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 1) | 0 | 33 | 54 | 65 | 87 | 93 | 95 |
| Milk + (100 ng/L) AFM1 + (1 g) bentonite (HAFR 3) | 0 | 37 | 60 | 80 | 93 | 95 | 98.5 |
| Samples | Mean (SD) | |||
|---|---|---|---|---|
| Fat | Protein | Lactose | SNF | |
| Milk (−Ve) | 0.55 b ± 0.005 | 3.57 b ± 0.001 | 4.91 a ± 0.015 | 8.49 c ± 0.004 |
| Milk +(100 ng/L) AFM1 (+ve) | 0.56 b ± 0.011 | 3.56 b ± 0.004 | 4.81 b ± 0.011 | 8.51 b ± 0.00 |
| Milk + (1 g) bentonite (HAFR 1) | 0.56 b ± 0.004 | 3.58 b ± 0.003 | 4.72 c ± 0.012 | 8.56 a ± 0.003 |
| Milk + (1 g) bentonite (HAFR 2) | 0.55 b ± 0.012 | 3.54 c ± 0.002 | 3.84 f ± 0.005 | 8.48 c ± 0.001 |
| Milk + (1 g) bentonite (HAFR 3) | 0.58 a ± 0.005 | 3.59 a ± 0.003 | 4.75 c ± 0.015 | 8.52 b ± 0.002 |
| Milk + (1 g) bentonite (HAFR 4) | 0.59 a ± 0.001 | 3.61 a ± 0.005 | 4.86 ab ± 0.004 | 8.59 a ± 0.011 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 1) | 0.54 c ± 0.011 | 3.53 c ± 0.004 | 4.83 b ± 0.013 | 8.54 a ± 0.012 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 2) | 0.53 c ± 0.002 | 3.56 b ± 0.012 | 4.67 d ± 0.02 | 8.56 a ± 0.003 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 3) | 0.56 b ± 0.001 | 3.54 c ± 0.011 | 4.72 c ± 0.003 | 8.55 a ± 0.004 |
| Milk + (100 ng/L) AFM1+ (1 g) bentonite (HAFR 4) | 0.58 a ± 0.016 | 3.58 b ± 0.011 | 4.51 e ± 0.004 | 8.56 a ± 0.006 |
| Concentration (µg/mL) | HAFR (1) | HAFR (2) | HAFR (3) | HAFR (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition % | Viability % | Inhibition % | Viability % | Inhibition % | Viability % | Inhibition % | Viability % | |
| 500 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| 250 | 85 | 15 | 98 | 2 | 99 | 1 | 98 | 2 |
| 125 | 72 | 28 | 92 | 8 | 97 | 3 | 91 | 9 |
| 62.5 | 62 | 38 | 88 | 12 | 94 | 6 | 87 | 13 |
| 31.25 | 44 | 56 | 73 | 27 | 86 | 14 | 77 | 23 |
| 15.6 | 29 | 71 | 56 | 44 | 79 | 21 | 71 | 29 |
| 7.8 | 16 | 84 | 41 | 59 | 66 | 34 | 58 | 42 |
| 3.9 | 8 | 92 | 36 | 64 | 51 | 49 | 52 | 48 |
| 1.95 | 6 | 94 | 21 | 79 | 42 | 58 | 43 | 57 |
| 0.97 | 2 | 98 | 10 | 90 | 6 | 94 | 34 | 66 |
| IC50 | 57.1 | 16.5 | 6.92 | 11.35 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, G.M.; El-Makarem, H.S.A.; Allam, M.G.; El Okle, O.S.; El-Toukhy, M.I.; Mehany, T.; El-Halmouch, Y.; Abushaala, M.M.F.; Saad, M.S.; Korma, S.A.; et al. Evaluation of the Adsorption Efficacy of Bentonite on Aflatoxin M1 Levels in Contaminated Milk. Toxins 2023, 15, 107. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins15020107
Hamad GM, El-Makarem HSA, Allam MG, El Okle OS, El-Toukhy MI, Mehany T, El-Halmouch Y, Abushaala MMF, Saad MS, Korma SA, et al. Evaluation of the Adsorption Efficacy of Bentonite on Aflatoxin M1 Levels in Contaminated Milk. Toxins. 2023; 15(2):107. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins15020107
Chicago/Turabian StyleHamad, Gamal M., Hussein S. Abo El-Makarem, Marwa G. Allam, Osama S. El Okle, Marwa I. El-Toukhy, Taha Mehany, Yasser El-Halmouch, Mukhtar M. F. Abushaala, Mohamed S. Saad, Sameh A. Korma, and et al. 2023. "Evaluation of the Adsorption Efficacy of Bentonite on Aflatoxin M1 Levels in Contaminated Milk" Toxins 15, no. 2: 107. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins15020107