Biosynthetic Pathways of Ergot Alkaloids
Abstract
:1. Introduction

2. Identification and Comparison of Biosynthetic Gene Clusters
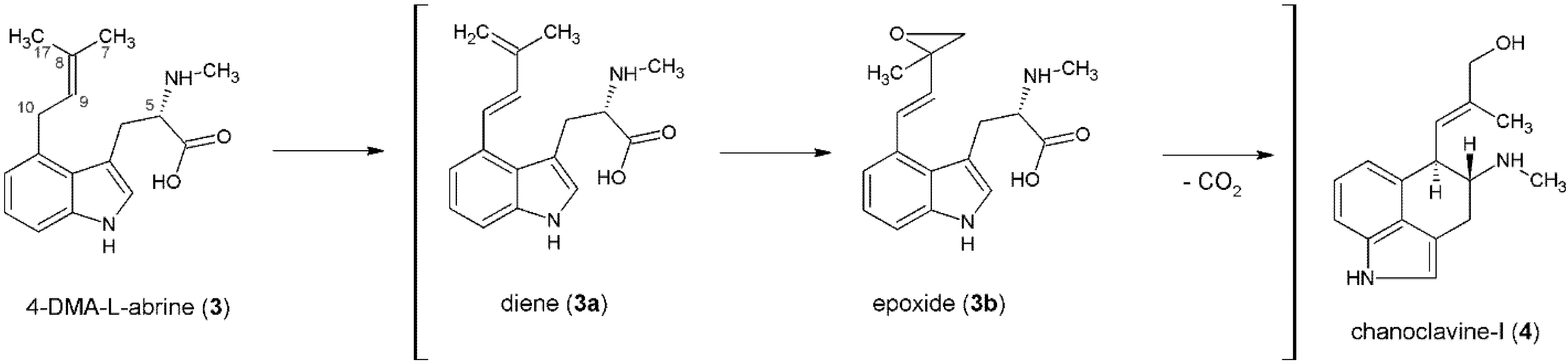
3. Formation of the Ergoline Ring—Common Steps
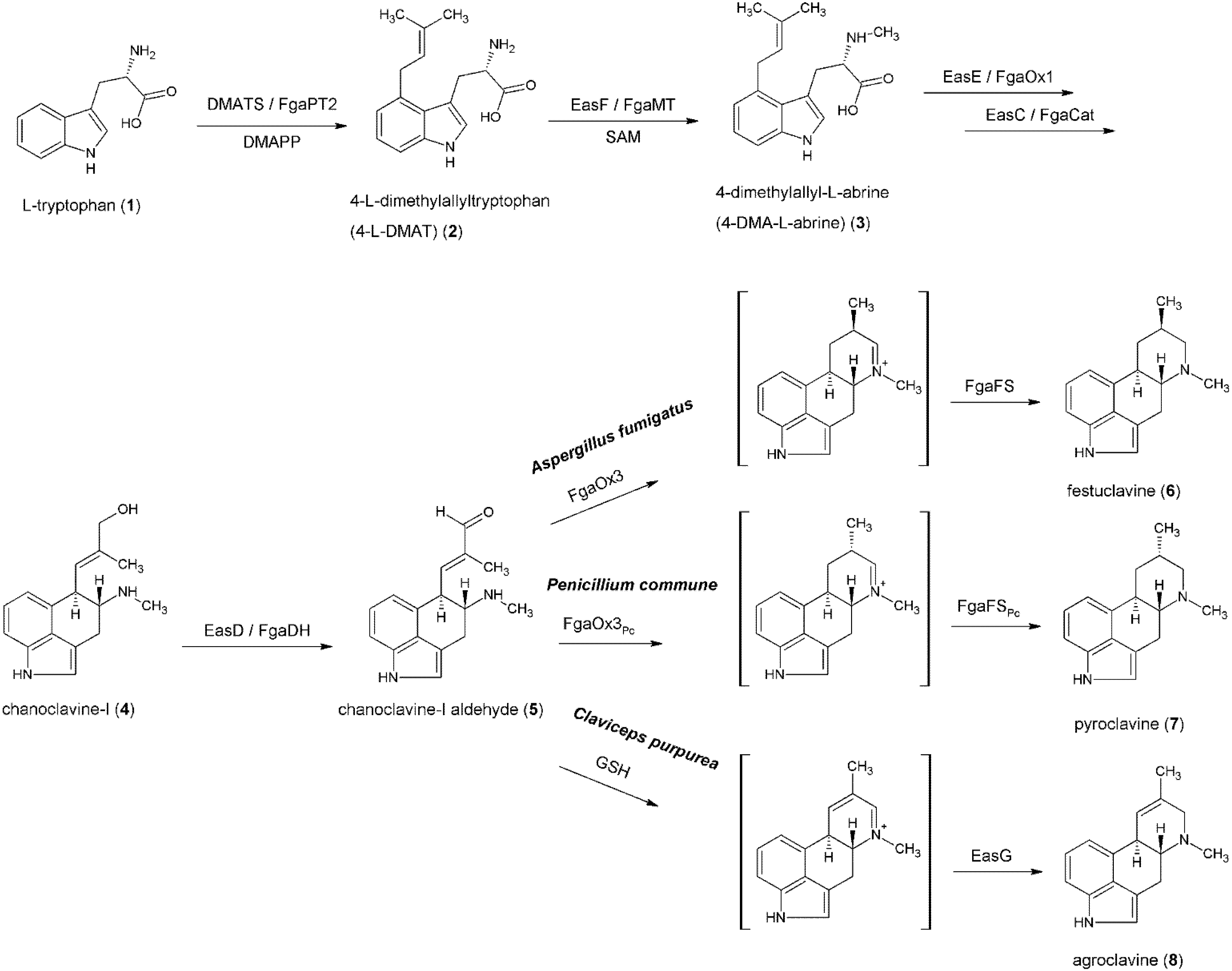
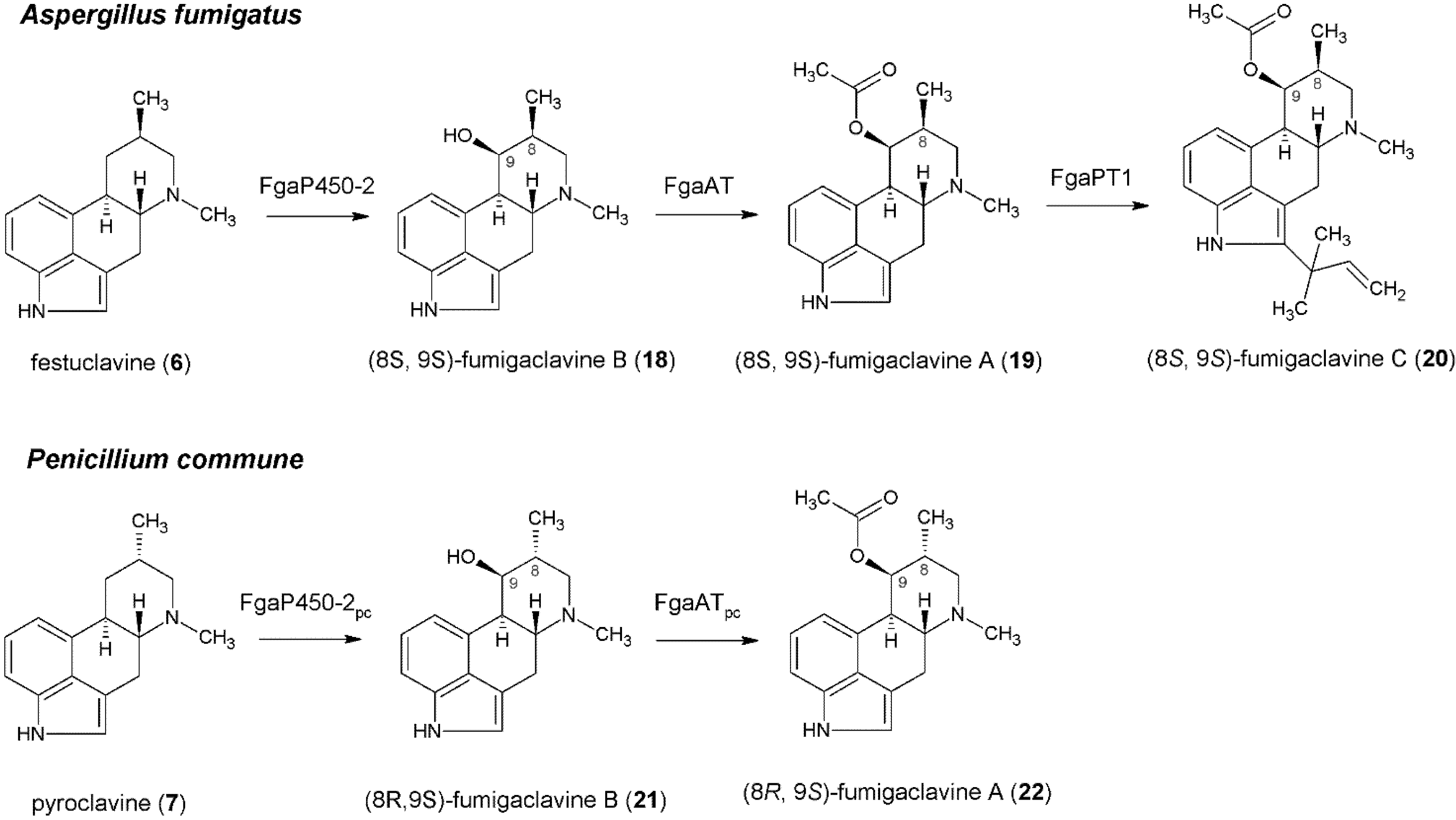
4. Formation of Fumigaclavines in Aspergillus fumigatus and Penicillium commune
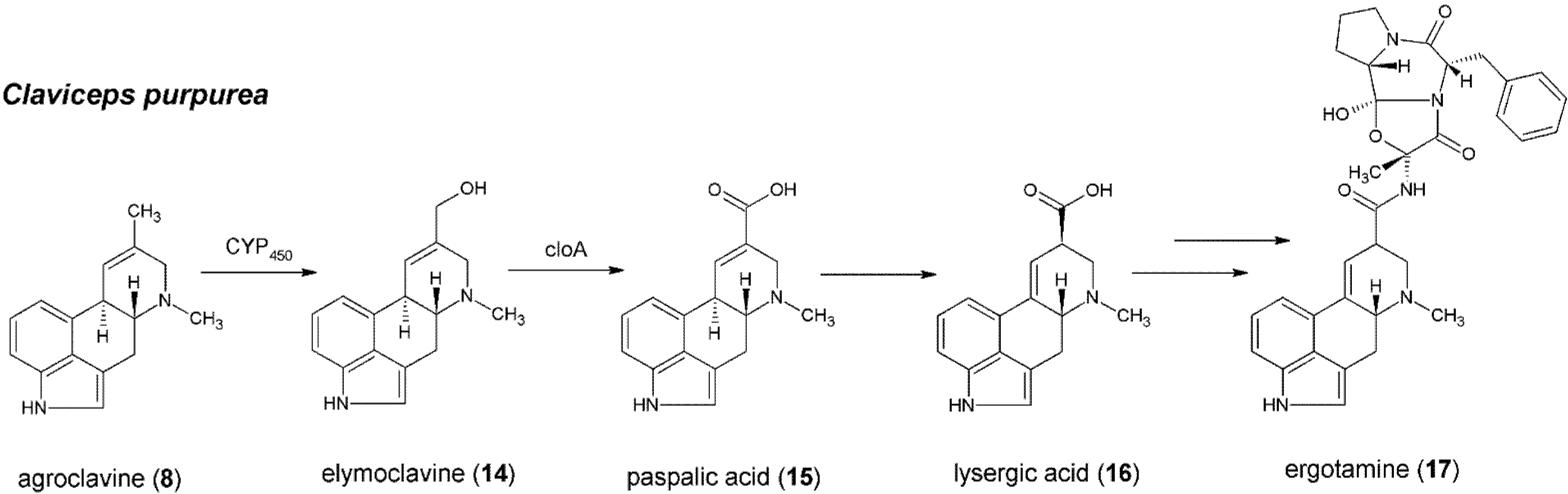
5. Formation of Lysergic Acid in Claviceps purpurea
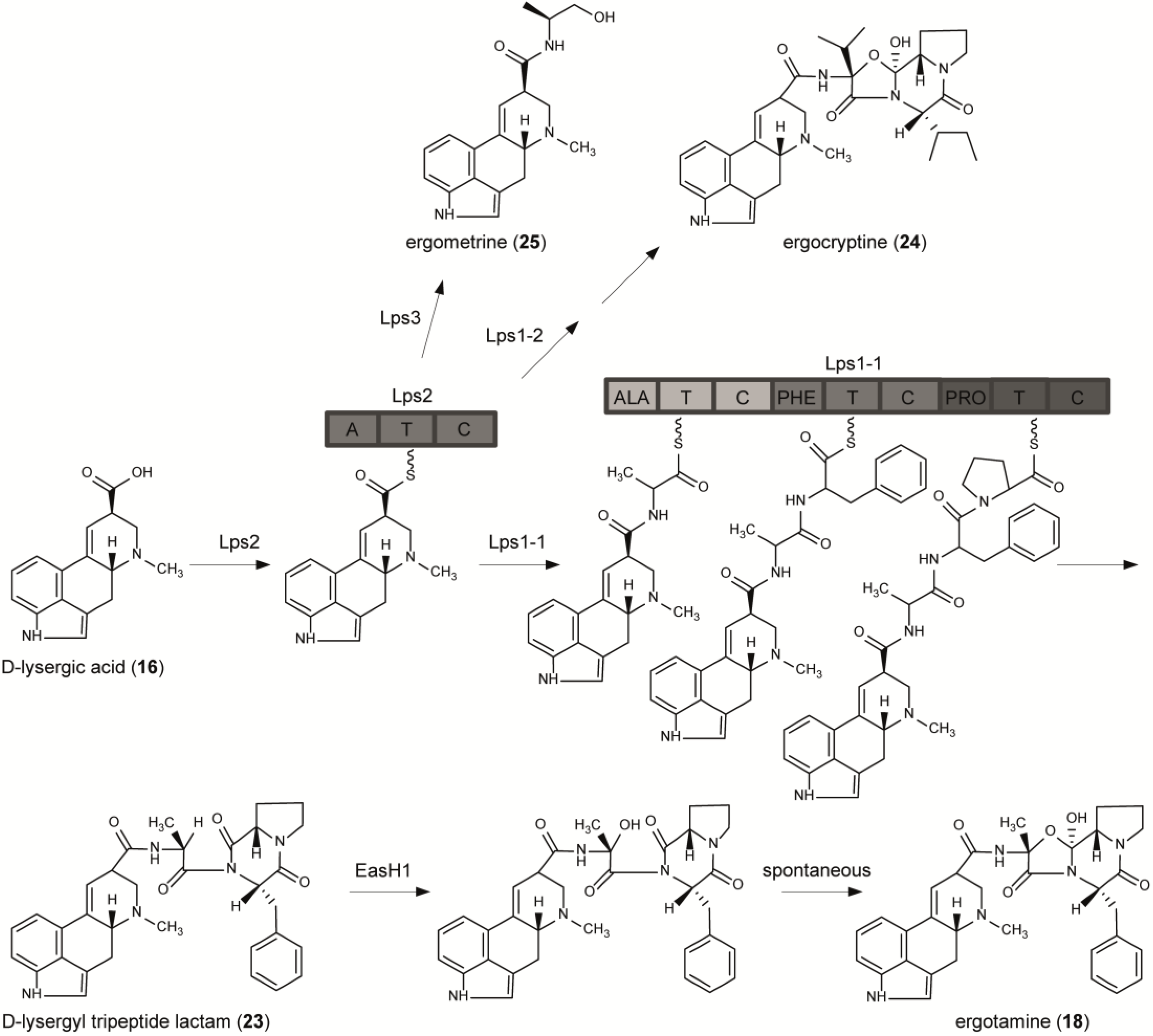
6. From Lysergic Acid to Ergoamides and Ergopeptines

7. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haarmann, T.; Rolke, Y.; Giesbert, S.; Tudzynski, P. Ergot: From witchcraft to biotechnology. Mol. Plant Pathol. 2009, 10, 563–577. [Google Scholar] [CrossRef]
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 1–10. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Cheng, J.Z.; O’Connor, S.E. Biosynthesis of the ergot alkaloids. Nat. Prod. Rep. 2014, 31, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Wallwey, C.; Li, S.M. Ergot alkaloids: Structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat. Prod. Rep. 2011, 28, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Ergot alkaloids—Biology and molecular biology. Alkaloids Chem. Biol. 2006, 63, 45–86. [Google Scholar] [PubMed]
- Boichenko, L.V.; Boichenko, D.M.; Vinokurova, N.G.; Reshetilova, T.A.; Arinbasarov, M.U. Screening for ergot alkaloid producers among microscopic fungi by means of the polymerase chain reaction. Microbiology 2001, 70, 306–310. [Google Scholar] [CrossRef]
- Hulvova, H.; Galuszka, P.; Frebortova, J.; Frebort, I. Parasitic fungus Claviceps as a source for biotechnological production of ergot alkaloids. Biotechnol. Adv. 2013, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Wilson, E.V.; Machado, C.; Schardl, C.L.; Tudzynski, P. Comparison of ergot alkaloid biosynthesis gene clusters in Claviceps species indicates loss of late pathway steps in evolution of C. fusiformis. Appl. Environ. Microbiol. 2007, 73, 7185–7191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G.; et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. acridum. PLoS. Genet. 2011. [Google Scholar] [CrossRef]
- Kozlovsky, A.G.; Zhelifonova, V.P.; Antipova, T.V.; Zelenkova, N.F. Physiological and biochemical characteristics of the genus Penicillium fungi as producers of ergot alkaloids and quinocitrinins. Appl. Biochem. Microbiol. 2011, 47, 426–430. [Google Scholar] [CrossRef]
- Ge, H.M.; Yu, Z.G.; Zhang, J.; Wu, J.H.; Tan, R.X. Bioactive alkaloids from endophytic Aspergillus fumigatus. J. Nat. Prod. 2009, 72, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.G.; Zhelifonova, V.P.; Antipova, T.V. Fungi of the genus Penicillium as producers of physiologically active compounds. Appl. Biochem. Microbiol. 2013, 49, 1–10. [Google Scholar]
- Wallwey, C.; Heddergott, C.; Xie, X.; Brakhage, A.A.; Li, S.M. Genome mining reveals the presence of a conserved gene cluster for the biosynthesis of ergot alkaloid precursors in the fungal family Arthrodermataceae. Microbiology 2012, 158, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, W.T.; Panaccione, D.G.; Hazekamp, C.S.; Mckee, M.C.; Ryan, K.L.; Clay, K. Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J. Chem. Ecol. 2013, 39, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Markert, A.; Steffan, N.; Ploss, K.; Hellwig, S.; Steiner, U.; Drewke, C.; Li, S.M.; Boland, W.; Leistner, E. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a Clavicipitalean fungus. Plant Physiol. 2008, 147, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Ahimsa-Müller, M.A.; Markert, A.; Hellwig, S.; Knoop, V.; Steiner, U.; Drewke, C.; Leistner, E. Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J. Nat. Prod. 2007, 70, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Pan, J.; Florea, S.; Takach, J.E.; Panaccione, D.G.; Farman, M.L.; Webb, J.S.; Jaromczyk, J.; Charlton, N.D.; et al. Currencies of mutualisms: Sources of alkaloid genes in vertically transmitted epichloae. Toxins 2013, 5, 1064–1088. [Google Scholar] [CrossRef] [PubMed]
- Gröger, D.; Floss, H.G. Biochemistry of ergot alkaloids—Achievements and challenges. Alkaloids Chem. Biol. 1998, 50, 171–218. [Google Scholar]
- Scandola, M.; Games, D.E.; Costa, C.; Allegri, G.; Bertazzo, A.; Curcuruto, O.; Traldi, P. Structural study of alkaloids from Securidaca longipedunculata roots II. Isolation and characterization by supercritical fluid chromatography/mass spectrometry. J. Heterocycl. Chem. 1994, 31, 219–224. [Google Scholar] [CrossRef]
- Li, S.M.; Unsöld, I.A. Post genome research on the biosynthesis of ergot alkaloids. Planta Med. 2006, 72, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Floss, H.G. Biosynthesis of ergot alkaloids and related compounds. Tetrahedron 1976, 32, 873–912. [Google Scholar]
- Williams, R.M.; Stocking, E.M.; Sanz-Cervera, J.F. Biosynthesis of prenylated alkaloids derived from tryptophan. Top. Curr. Chem. 2000, 209, 97–173. [Google Scholar]
- Gebler, J.C.; Poulter, C.D. Purification and characterization of dimethylallyl tryptophan synthase from Claviceps purpurea. Arch. Biochem. Biophys. 1992, 296, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Wang, H.; Gebler, J.C.; Poulter, C.D.; Schardl, C.L. The Claviceps purpurea gene encoding dimethylallyltryptophan synthase, the committed step for ergot alkaloid biosynthesis. Biochem. Biophys. Res. Commun. 1995, 216, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, P.; Holter, K.; Correia, T.; Arntz, C.; Grammel, N.; Keller, U. Evidence for an ergot alkaloid gene cluster in Claviceps purpurea. Mol. Gen. Genet. 1999, 261, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, T.; Machado, C.; Lübbe, Y.; Correia, T.; Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. The ergot alkaloid gene cluster in Claviceps purpurea: Extension of the cluster sequence and intra species evolution. Phytochemistry 2005, 66, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Haarmann, T.; Pazoutova, S.; Jung, M.; Tudzynski, P. The ergot alkaloid gene cluster: Functional analyses and evolutionary aspects. Phytochemistry 2009, 70, 1822–1932. [Google Scholar] [CrossRef]
- Haarmann, T.; Lorenz, N.; Tudzynski, P. Use of a nonhomologous end joining deficient strain (Deltaku70) of the ergot fungus Claviceps purpurea for identification of a nonribosomal peptide synthetase gene involved in ergotamine biosynthesis. Fungal Genet. Biol. 2008, 45, 35–44. [Google Scholar] [CrossRef]
- Fleetwood, D.J.; Scott, B.; Lane, G.A.; Tanaka, A.; Johnson, R.D. A complex ergovaline gene cluster in epichloe endophytes of grasses. Appl. Environ. Microbiol. 2007, 73, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Johnson, R.D.; Wang, J.; Young, C.A.; Damrongkool, P.; Scott, B.; Schardl, C.L. Elimination of ergovaline from a grass-Neotyphodium endophyte symbiosis by genetic modification of the endophyte. Proc. Natl. Acad. Sci. USA 2001, 98, 12820–12825. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Coyle, C.M. Abundant respirable ergot alkaloids from the common airborne fungus Aspergillus fumigatus. Appl. Environ. Microbiol. 2005, 71, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Unsöld, I.A. Molecular Biological and Biochemical Investigations on the Biosynthesis of Fumigaclavines in Aspergillus fumigatus AF 293/B 5233 and Penicillium commune NRRL2033. Ph.D. Thesis, Universität Tübingen, Tübingen, German, 2006. [Google Scholar]
- Lee, S.L.; Floss, H.G.; Heinstein, P. Purification and properties of dimethylallylpyrophosphate: Tryptophan dimethylallyl transferase, the first enzyme of ergot alkaloid biosynthesis in Claviceps. sp. SD 58. Arch. Biochem. Biophys. 1976, 177, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.M.; Panaccione, D.G. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 2005, 71, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Unsöld, I.A.; Li, S.M. Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 2005, 151, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Steffan, N.; Unsöld, I.A.; Li, S.M. Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 2007, 8, 1298–1307. [Google Scholar] [CrossRef]
- Steffan, N.; Li, S.M. Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch. Microbiol. 2009, 191, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Metzger, U.; Schall, C.; Zocher, G.; Unsöld, I.; Stec, E.; Li, S.-M.; Heide, L.; Stehle, T. The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 14309–14314. [Google Scholar] [CrossRef] [PubMed]
- Luk, L.Y.P.; Tanner, M.E. Mechanism of dimethylallyltryptophan synthase: Evidence for a dimethylallyl cation intermediate in an aromatic prenyltransferase reaction. J. Am. Chem. Soc. 2009, 131, 13932–13933. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Panaccione, D.G.; Schardl, C.L. Phylogenetic analyses reveal monophyletic origin of the ergot alkaloid gene dmaW in fungi. Evol. Bioinform. 2009, 5, 15–30. [Google Scholar]
- Yu, X.; Li, S.M. Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol. 2012, 516, 259–278. [Google Scholar] [PubMed]
- Liebhold, M.; Xie, X.; Li, S.-M. Expansion of enzymatic Friedel-Crafts alkylation on indoles: Acceptance of unnatural beta-unsaturated allyl diphospates by dimethylallyl-tryptophan synthases. Org. Lett. 2012, 14, 4884–4885. [Google Scholar] [CrossRef]
- Liebhold, M.; Li, S.M. Regiospecific benzylation of tryptophan and derivatives catalyzed by a fungal dimethylallyl transferase. Org. Lett. 2013, 15, 5834–5837. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products. Appl. Microbiol. Biotechnol. 2009, 84, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Rigbers, O.; Li, S.M. Ergot alkaloid biosynthesis in Aspergillus fumigatus: Overproduction and biochemical characterisation of a 4-dimethylallyltryptophan N-methyltransferase. J. Biol. Chem. 2008, 283, 26859–26868. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Olšovská, J.; Šulc, M.; Tudzynski, P. The alkaloid cluster gene ccsA of the ergot fungus Claviceps purpurea encodes the chanoclavine-I-synthase, an FAD-containing oxidoreductase mediating the transformation of N-methyl-dimethylallyltryptophan to chanoclavine-I. Appl. Environ. Microbiol. 2010, 76, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Goetz, K.E.; Coyle, C.M.; Cheng, J.Z.; O’Connor, S.E.; Panaccione, D.G. Ergot cluster-encoded catalase is required for synthesis of chanoclavine-I in Aspergillus fumigatus. Curr. Genet. 2011, 57, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Chen, C.; Wu, J.P.; Shibuya, M.; Kim, C.G.; Floss, H.G. Probing ergot alkaloid biosynthesis: Intermediates in the formation of ring C. J. Am. Chem. Soc. 1993, 115, 2482–2488. [Google Scholar] [CrossRef]
- Ryan, K.L.; Moore, C.T.; Panaccione, D.G. Partial reconstruction of the ergot alkaloid pathway by heterologous gene expression in Aspergillus nidulans. Toxins 2013, 5, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Folly, C.; Hatsch, A.; Molt, A.; Schroder, H.; O’Connor, S.E.; Naesby, M. The important ergot alkaloid intermediate chanoclavine-I produced in the yeast Saccharomyces cerevisiae by the combined action of EasC and EasE from Aspergillus japonicus. Microb. Cell Fact. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Wallwey, C.; Matuschek, M.; Li, S.M. Ergot alkaloid biosynthesis in Aspergillus fumigatus: Conversion of chanoclavine-I to chanoclavine-I aldehyde catalyzed by a short-chain alcohol dehydrogenase FgaDH. Arch. Microbiol. 2010, 192, 127–134. [Google Scholar] [CrossRef]
- Matuschek, M.; Wallwey, C.; Wollinsky, B.; Xie, X.; Li, S.M. In vitro conversion of chanoclavine-I aldehyde to the stereoisomers festuclavine and pyroclavine controlled by the second reduction step. RSC Adv. 2012, 2, 3662–3669. [Google Scholar] [CrossRef]
- Coyle, C.M.; Cheng, J.Z.; O’Connor, S.E.; Panaccione, D.G. An old yellow enzyme gene that controls the branch point between Aspergillus fumigatus and Claviceps purpurea ergot alkaloid pathways. Appl. Environ. Microbiol. 2010, 76, 3898–3903. [Google Scholar] [CrossRef] [PubMed]
- Wallwey, C.; Matuschek, M.; Xie, X.L.; Li, S.M. Ergot alkaloid biosynthesis in Aspergillus fumigatus: Conversion of chanoclavine-I aldehyde to festuclavine by the festuclavine synthase FgaFS in the presence of the old yellow enzyme FgaOx3. Org. Biomol. Chem. 2010, 8, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wallwey, C.; Matuschek, M.; Steinbach, K.; Li, S.M. Formyl migration product of chanoclavine-I aldehyde in the presence of the old yellow enzyme FgaOx3 from Aspergillus fumigatus: A NMR structure elucidation. Magn Reson. Chem. 2011, 49, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Z.; Coyle, C.M.; Panaccione, D.G.; O’Connor, S.E. Controlling a structural branch point in ergot alkaloid biosynthesis. J. Am. Chem. Soc. 2010, 132, 12835–12837. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, M.; Wallwey, C.; Xie, X.; Li, S.M. New insights into ergot alkaloid biosynthesis in Claviceps purpurea: An agroclavine synthase EasG catalyses, via a non-enzymatic adduct with reduced glutathione, the conversion of chanoclavine-I aldehyde to agroclavine. Org. Biomol. Chem. 2011, 9, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Steffan, N.; Yin, W.B.; Li, S.M. Ergot alkaloid biosynthesis in Aspergillus fumigatus: FgaAT catalyses the acetylation of fumigaclavine B. ChemBioChem 2009, 10, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Unsöld, I.A.; Li, S.M. Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: Gene expression, purification and characterization of fumigaclavine C synthase FgaPT1. ChemBioChem 2006, 7, 158–164. [Google Scholar] [CrossRef]
- O’Hanlon, K.A.; Gallagher, L.; Schrettl, M.; Jochl, C.; Kavanagh, K.; Larsen, T.O.; Doyle, S. Nonribosomal peptide synthetase genes pesL and pes1 are essential for fumigaclavine C production in Aspergillus fumigatus. Appl. Environ. Microbiol. 2012, 78, 3166–3176. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Schumann, B.; Gröger, D. Microsomal oxygenases involved in ergoline alkaloid biosynthesis of various Claviceps strains. J. Basic Microbiol. 1988, 28, 83–93. [Google Scholar] [CrossRef]
- Kim, S.U.; Cho, Y.J.; Floss, H.G.; Anderson, J.A. Conversion of elymoclavine to paspalic acid by a particulate fraction from an ergotamine-producing strain of Claviceps sp. Planta Med. 1983, 48, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, T.; Ortel, I.; Tudzynski, P.; Keller, U. Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. ChemBioChem 2006, 7, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Panaccione, D.G. Heterologous expression of lysergic acid and novel ergot alkaloids in Aspergillus fumigatus. Appl. Environ. Microbiol. 2014. [Google Scholar] [CrossRef]
- Riederer, B.; Han, M.; Keller, U. d-Lysergyl peptide synthetase from the ergot fungus Claviceps purpurea. J. Biol. Chem. 1996, 271, 27524–27530. [Google Scholar] [CrossRef] [PubMed]
- Walzel, B.; Riederer, B.; Keller, U. Mechanism of alkaloid cyclopeptide synthesis in the ergot fungus Claviceps purpurea. Chem. Biol. 1997, 4, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.; Grammel, N.; Ortel, I.; Keller, U.; Tudzynski, P. Molecular cloning and analysis of the ergopeptine assembly system in the ergot fungus Claviceps purpurea. Chem. Biol. 2003, 10, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ortel, I.; Keller, U. Combinatorial assembly of simple and complex d-lysergic acid alkaloid peptide classes in the ergot fungus Claviceps purpurea. J. Biol. Chem. 2009, 284, 6650–6660. [Google Scholar] [CrossRef] [PubMed]
- Havemann, J.; Vogel, D.; Loll, B.; Keller, U. Cyclolization of d-lysergic acid alkaloid peptides. Chem. Biol. 2014, 21, 146–155. [Google Scholar]
- Keller, U.; Tudzynski, P. Ergot alkaloids. In Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Tudzynski, P.; Neubauer, L. Ergot alkaloids. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Springer: New York, NY, USA, 2014; pp. 303–316. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins 2014, 6, 3281-3295. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6123281
Gerhards N, Neubauer L, Tudzynski P, Li S-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins. 2014; 6(12):3281-3295. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6123281
Chicago/Turabian StyleGerhards, Nina, Lisa Neubauer, Paul Tudzynski, and Shu-Ming Li. 2014. "Biosynthetic Pathways of Ergot Alkaloids" Toxins 6, no. 12: 3281-3295. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6123281



