A Facile Preparation and Energetic Characteristics of the Core/Shell CoFe2O4/Al Nanowires Thermite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
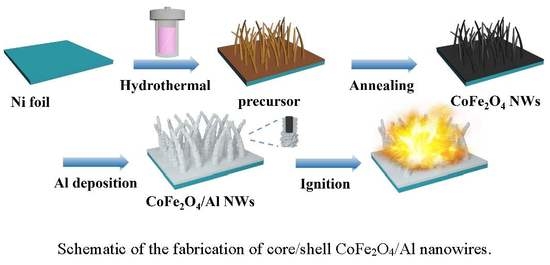
2.2. Synthesis of the CoFe2O4 NWs Film
2.3. Synthesis of the CoFe2O4/Al NWs Nanothermite Film
2.4. Characterizations
3. Results and Discussion
Co(OH)z(CO3)0.5(2-z)·2Fe(OH)3·nH2O + (x+2y)F−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly reactivemetastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, e1706293. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials:synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Khasainov, B.; Comet, M.; Veyssiere, B.; Spitzer, D. Comparison of performance of fast-reacting nanothermites and primary explosives. Propellants Explos. Pyrotech. 2017, 42, 754–772. [Google Scholar] [CrossRef]
- Dong, Z.; Al-Sharab, J.F.; Kear, B.H.; Tse, S.D. Combined flame and electrodeposition synthesis of energetic coaxial tungsten-oxide/aluminum nanowire arrays. Nano Lett. 2013, 13, 4346–4350. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, J.; Du, Y.; Zhang, P.; Li, S.; Fang, T.; Pang, S. New roles of metal-organic frameworks: Fuels for aluminum-free energetic thermites with low ignition temperatures, high peak pressures and high activity. Combust. Flame 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Kline, D.J.; Eckman, N.; Agrawal, N.R.; Wu, T.; Wang, P.; Zachariah, M.R. Direct writing of a 90wt% particle loading nanothermite. Adv. Mater. 2019, 31, e1806575. [Google Scholar] [CrossRef]
- Jacob, R.J.; Hill, K.J.; Yang, Y.; Pantoya, M.L.; Zachariah, M.R. Pre-stressing aluminum nanoparticles as a strategy to enhance reactivity of nanothermite composites. Combust. Flame 2019, 205, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Yang, Y.; Dong, Y.; Wang, L.; Chen, X.; Zhang, J.; He, J. Reaction products and their solidification process of the plasma sprayed Fe2O3-Al composite powders. Mater. Chem. Phys. 2012, 133, 190–196. [Google Scholar] [CrossRef]
- Comet, M.; Vidick, G.; Schnell, F.; Suma, Y.; Baps, B.; Spitzer, D. Sulfates-based nanothermites: An expanding horizon for metastable interstitial composites. Angew. Chem. Int. Ed. 2015, 54, 4458–4462. [Google Scholar] [CrossRef]
- Elbasuney, S. Novel colloidal nanothermite particles (MnO2/Al) for advanced highly energetic systems. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1793–1800. [Google Scholar] [CrossRef]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.-J.; Yan, Q.-L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermite foams: From nanopowder to object. Chem. Eng. J. 2017, 316, 807–812. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Shu, Y.; Guo, X.; Bao, H.; Li, W.; Zhu, Y.; Li, Y.; Huang, X. Fabrication of electrophoretically deposited, self-assembled three-dimensional porous Al/CuO nanothermite films for highly enhanced energy output. Mater. Chem. Phys. 2017, 194, 182–187. [Google Scholar] [CrossRef]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.; Zhang, K. Nanostructured energetic composites: Synthesis, ignition/combustion modeling, and applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef]
- Xu, J.; Shen, Y.; Wang, C.; Dai, J.; Tai, Y.; Ye, Y.; Shen, R.; Wang, H.; Zachariah, M.R. Controlling the energetic characteristics of micro energy storage device by in situ deposition Al/MoO3 nanolaminates with varying internal structure. Chem. Eng. J. 2019, 373, 345–354. [Google Scholar] [CrossRef]
- Luo, Q.; Long, X.; Nie, F.; Liu, G.; Zhu, M. The safety properties of a potential kind of novel green primary explosive: Al/Fe2O3/RDX nanocomposite. Materials 2018, 11, 1930. [Google Scholar] [CrossRef] [Green Version]
- Glavier, L.; Nicollet, A.; Jouot, F.; Martin, B.; Barberon, J.; Renaud, L.; Rossi, C. Nanothermite/RDX-based miniature device for impact ignition of high explosives. Propellants Explos. Pyrotech. 2017, 42, 308–317. [Google Scholar] [CrossRef]
- Wang, A.; Bok, S.; Thiruvengadathan, R.; Gangopadhyay, K.; McFarland, J.A.; Maschmann, M.R.; Gangopadhyay, S. Reactive nanoenergetic graphene aerogel synthesized by one-step chemical reduction. Combust. Flame 2018, 196, 400–406. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, S.B.; Kim, J.H.; Jang, N.S.; Kim, D.H.; Lee, H.W.; Kim, J.M.; Kim, S.H. A micro-chip initiator with controlled combustion reactivity realized by integrating Al/CuO nanothermite composites on a microhotplate platform. J. Micromech. Microeng. 2015, 26, 015002. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, W.D.; Lee, S.; Lee, D.C. Nanothermite of Al nanoparticles and three-dimensionally ordered macroporous CuO: Mechanistic insight into oxidation during thermite reaction. Combust. Flame 2018, 189, 87–91. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Yang, G.; Zhang, Q.; Shen, J.; Lu, J.; Zhang, K. Highly exothermic and superhydrophobic Mg/fluorocarbon core/shell nanoenergetic arrays. ACS Appl. Mater. Interfaces 2014, 6, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, Z.; Yang, Y.; Shen, J.; Long, Z.; Li, Z.; Cui, X.; Yang, G. Core-shell Al-polytetrafluoroethylene (PTFE) configurations to enhance reaction kinetics and energy performance for nanoenergetic materials. Chemistry 2016, 22, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, D.G.; Lu, J.; Zhang, K.L. CuO/Mg/fluorocarbon sandwich-structure superhydrophobic nanoenergetic composite with anti-humidity property. Chem. Eng. J. 2015, 266, 163–170. [Google Scholar] [CrossRef]
- Petrantoni, M.; Rossi, C.; Salvagnac, L.; Conédéra, V.; Estève, A.; Tenailleau, C.; Alphonse, P.; Chabal, Y.J. Multilayered Al/CuO thermite formation by reactive magnetron sputtering: Nano versus micro. J. Appl. Phys. 2010, 108, 084323. [Google Scholar] [CrossRef] [Green Version]
- Taton, G.; Lagrange, D.; Conedera, V.; Renaud, L.; Rossi, C. Micro-chip initiator realized by integrating Al/CuO multilayer nanothermite on polymeric membrane. J. Micromech. Microeng. 2013, 23, 105009. [Google Scholar] [CrossRef]
- Nicollet, A.; Lahiner, G.; Belisario, A.; Souleille, S.; Djafari-Rouhani, M.; Estève, A.; Rossi, C. Investigation of Al/CuO multilayered thermite ignition. J. Appl. Phys. 2017, 121, 034503. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Zhang, W.; Hu, B.; Ni, D.; Zheng, Z.; Liu, J.; Ma, K.; Ren, W. Core/shell CuO/Al nanorod thermite film based on electrochemical anodization. Nanotechnology 2018, 29, 36LT02. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.J.; Cheng, Z.P.; Ke, X.; Jiang, W. Facile preparation and energetic characteristics of core-shell Al/CuO metastable intermolecular composite thin film on a silicon substrate. Chem. Eng. J. 2017, 328, 585–590. [Google Scholar] [CrossRef]
- Ohkura, Y.; Liu, S.-Y.; Rao, P.M.; Zheng, X. Synthesis and ignition of energetic CuO/Al core/shell nanowires. Proc. Combust. Inst. 2011, 33, 1909–1915. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Xu, J.B.; Ma, X.X.; Ye, Y.H.; Yang, G.C.; Zhang, K.L. In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem. Eng. J. 2018, 354, 885–895. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.; Shen, J.; Li, R.; Yang, Y.; Yang, G. Large-Scale synthesis of a porous Co3O4 nanostructure and its application in metastable intermolecular composites. Propellants Explos. Pyrotech. 2015, 40, 514–517. [Google Scholar] [CrossRef]
- Zhang, K.; Rossi, C.; Tenailleau, C.; Alphonse, P.; Chane-Ching, J.-Y. Synthesis of large-area and aligned copper oxide nanowires from copper thin film on silicon substrate. Nanotechnology 2007, 18, 275607. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, W.; Gao, Y.; Ni, D.; Ye, J.; Zhu, C.; Ma, K. The super-hydrophobic thermite film of the Co3O4/Al core/shell nanowires for an underwater ignition with a favorable aging-resistance. Chem. Eng. J. 2018, 338, 99–106. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yu, C.; Ni, D.; Ma, K.; Ye, J. Controllable synthesis of NiCo2O4 /Al core-shell nanowires thermite film with excellent heat release and short ignition time. Mater. Des. 2018, 155, 396–403. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J.; Huang, X.; Li, Y.; Ding, R.; Ji, X.; Hu, Y.; Chi, Q.; Zhu, Z. General synthesis of large-scale arrays of one-dimensional nanostructured Co3O4 directly on heterogeneous substrates. Cryst. Growth Des. 2010, 10, 70–75. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, W.; Gao, Y.; Chen, Y.; Ma, K.; Ye, J.; Shen, R.; Yang, Y. Shape-controlled syntheses of Co3O4 nanowires arrays with excellent catalytic performances upon ammonium perchlorate decomposition. Mater. Res. Bull. 2018, 97, 483–489. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Y.; Cheng, H.; Li, Y.Y.; Zhang, K. Integration of nano-Al with Co3O4 nanorods to realize high-exothermic core-shell nanoenergetic materials on a silicon substrate. Combust. Flame 2012, 159, 2202–2209. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Li, G.; Luo, Y. Tuning the reactivity of Al/Fe2O3 nanoenergetic materials via an approach combining soft template self-assembly with sol–gel process process. J. Solid State Chem. 2015, 230, 1–7. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, W.; Yu, C.; Zheng, G.; Ma, K.; Qin, Z.; Ye, J.; Chao, Y. Integration of the 3DOM Al/Co3O4 nanothermite film with a semiconductor bridge to realize a high-output micro-energetic igniter. RSC Adv. 2018, 8, 2552–2560. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhang, W.; Cheng, J.; Yu, C.; Shen, R.; Ye, J.; Qin, Z.; Chao, Y. A high energy output and low onset temperature nanothermite based on three-dimensional ordered macroporous nano-NiFe2O4. RSC Adv. 2016, 6, 93330–93334. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Ren, W.; Wu, G.; Zhang, W.; Hu, B.; Ni, D.; Zheng, Z.; Ma, K.; Ye, J.; Zhu, C. A Facile Preparation and Energetic Characteristics of the Core/Shell CoFe2O4/Al Nanowires Thermite Film. Micromachines 2020, 11, 516. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11050516
Yu C, Ren W, Wu G, Zhang W, Hu B, Ni D, Zheng Z, Ma K, Ye J, Zhu C. A Facile Preparation and Energetic Characteristics of the Core/Shell CoFe2O4/Al Nanowires Thermite Film. Micromachines. 2020; 11(5):516. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11050516
Chicago/Turabian StyleYu, Chunpei, Wei Ren, Ganggang Wu, Wenchao Zhang, Bin Hu, Debin Ni, Zilong Zheng, Kefeng Ma, Jiahai Ye, and Chenguang Zhu. 2020. "A Facile Preparation and Energetic Characteristics of the Core/Shell CoFe2O4/Al Nanowires Thermite Film" Micromachines 11, no. 5: 516. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11050516