Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib
Abstract
:1. Introduction
2. Results
2.1. Comparison of Baseline Characteristics and Laboratory Data of The Sorafenib-Treated HCC Patients with and without Sarcopenia
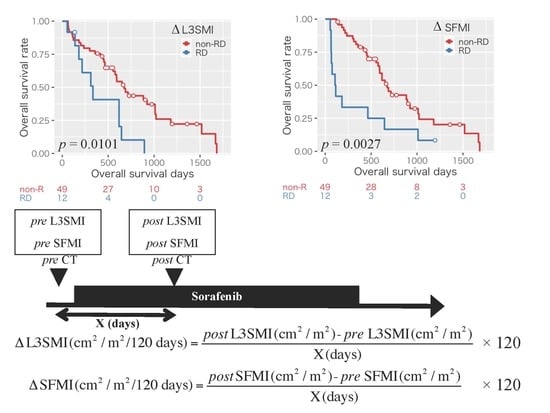
2.2. Impact of Sarcopenia and the Changes in Body Composition on Overall Survival in the Patients with HCC Treated with Sorafenib
3. Discussion
4. Materials and Methods
4.1. Patients, Treatment, and Follow-up Strategy
4.2. Image Analysis of Skeletal Muscle Mass and Subcutaneous and Visceral Fat Mass
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Njei, B.; Rotman, Y.; Ditah, I.; Lim, J.K. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015, 61, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; El-Serag, H.B. Hepatocellular Carcinoma from Epidemiology to Prevention: Translating Knowledge into Practice. Clin. Gastroenterol. Hepatol. 2015, 13, 2140–2151. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma: An epidemiologic view. J. Clin. Gastroenterol. 2002, 35, S72–S78. [Google Scholar] [CrossRef]
- Poon, R.T. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology 2011, 54, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Ku, Y.; Kaneko, S.; Okazaki, M.; Matsuyama, Y.; Igaki, H.; Kawasaki, S.; Tateishi, R.; Akahane, M.; Kudo, M.; et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol. Res. 2015, 45. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Schütte, K.; Tippelt, B.; Schulz, C.; Röhl, F.W.; Feneberg, A.; Seidensticker, R.; Arend, J.; Malfertheiner, P. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC). Clin. Nutr. 2015, 34, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Iritani, S.; Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M.; Moriwaki, H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Kochi, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int. J. Mol. Sci. 2015, 16, 9612–9624. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kawai, H.; Nakano, O.; Abe, S.; Kamimura, H.; Sakamaki, A.; Kamimura, K.; Tsuchiya, A.; Takamura, M.; Yamagiwa, S.; et al. Prognostic value of subcutaneous adipose tissue volume in hepatocellular carcinoma treated with transcatheter intra-arterial therapy. Cancer Manag. Res. 2018, 10, 2231–2239. [Google Scholar] [CrossRef]
- Itoh, S.; Shirabe, K.; Matsumoto, Y.; Yoshiya, S.; Muto, J.; Harimoto, N.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Nishie, A.; et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 3063–3068. [Google Scholar] [CrossRef]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Kawano, R.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; et al. No muscle depletion with high visceral fat as a novel beneficial biomarker of sorafenib for hepatocellular carcinoma. Liver Cancer 2018, 7, 359–371. [Google Scholar] [CrossRef]
- Nault, J.C.; Pigneur, F.; Nelson, A.C.; Costentin, C.; Tselikas, L.; Katsahian, S.; Diao, G.; Laurent, A.; Mallat, A.; Duvoux, C.; et al. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig. Liver Dis. 2015, 47, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Tanaka, T.; Moriwaki, H. Obesity and hepatocellular carcinoma: Targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin. Immunopathol. 2013, 35, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Sarcopenia Impairs Prognosis of Patients with Hepatocellular Carcinoma: The Role of Liver Functional Reserve and Tumor-Related Factors in Loss of Skeletal Muscle Volume. Nutrients 2017, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Baracos, V.E.; Antoun, S.; Birdsell, L.; Sawyer, M.B.; Venner, P. Association of Skeletal Muscle Wasting with Treatment with Sorafenib in Patients with Advanced Renal Cell Carcinoma: Results from a Placebo-Controlled Study. J. Clin. Oncol. 2010, 28, 1054–1060. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mechanisms to explain wasting of muscle and fat in cancer cachexia. Curr. Opin. Support. Palliat. Care 2007. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, R.A.; Wilke, M.S.; Perrine, M.; Pawlowicz, M.; Mourtzakis, M.; Lieffers, J.R.; Maneshgar, M.; Bruera, E.; Clandinin, M.T.; Baracos, V.E.; et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 2010, 29, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, K.; Takai, K.; Hanai, T.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; Moriwaki, H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-ROM test. J. Cancer Res. Clin. Oncol. 2013, 139, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Nishigaki, Y.; Shimizu, S.; Naiki, T.; Hayashi, H.; Uematsu, T.; Sugihara, J.; Tomita, E.; Shimizu, M.; et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol. Res. 2010, 40, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Takai, K.; Imai, K.; Shimizu, M.; Naiki, T.; Nagaki, M.; Moriwaki, H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr. 2011, 49, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Takai, K.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. Int. J. Mol. Sci. 2019, 20, 605. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Shiraki, M.; Fukushima, H.; Shimizu, M.; Iwasa, J.; Naiki, T.; Nagaki, M. Long-term outcome of branched-chain amino acid treatment in patients with liver cirrhosis. Hepatol. Res. 2008, 38 (Suppl. 1), S102–S106. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Maeda, T.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget 2018, 9, 14058–14067. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Takayasu, K.; Furuse, J.; Nakamura, K.; Tanaka, M.; Kudo, M.; Ikai, I.; Kubo, S.; Sakamoto, M.; Makuuchi, M. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol. Res. 2010, 40, 686–692. [Google Scholar] [CrossRef]
- Arizumi, T.; Ueshima, K.; Takeda, H.; Osaki, Y.; Takita, M.; Inoue, T.; Kitai, S.; Yada, N.; Hagiwara, S.; Minami, Y.; et al. Comparison of systems for assessment of post-therapeutic response to sorafenib for hepatocellular carcinoma. J. Gastroenterol. 2014, 49, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Mitsiopoulos, N.; Ross, R.; Gallagher, D.; Lyons, W.; Baumgartner, R.N.; Heymsfield, S.B. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 2017, 85, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Waliul Islam, A.H.M.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef]




| Variables | All Cases (n = 61) | Non-Sarcopenia (n = 36) | Sarcopenia (n = 25) | p-Value | |
|---|---|---|---|---|---|
| Sex (male/female) | 54/7 | 32/4 | 22/3 | >0.999 | |
| Age (years) | 67.3 ± 11.5 | 67.1 ± 11.7 | 67.7 ± 11.3 | 0.826 | |
| Etiology (B/C/others) | 14/28/19 | 6/17/13 | 8/11/6 | 0.504 | |
| Child-Pugh score (5/6) | 43/18 | 27/9 | 16/9 | 0.401 | |
| Stage (III/IVA/IVB) | 20/13/28 | 11/10/15 | 9/3/13 | 0.348 | |
| Combination therapy (yes/no) | 42/19 | 24/12 | 18/7 | 0.781 | |
| BMI (kg/m2) | 22.3 ± 3.0 | 23.9 ± 2.4 | 20.1 ± 2.4 | <0.001 | |
| L3SMI (cm2/m2) | All | 44.0 ± 7.7 | 48.5 ± 6.1 | 37.5 ± 4.6 | <0.001 |
| Male | 44.5 ± 7.7 | 48.9 ± 6.2 | 38.2 ± 4.4 | <0.001 | |
| Female | 39.9 ± 7.4 | 45.2 ± 4.3 | 32.7 ± 2.3 | 0.006 | |
| SFMI (cm2/m2) | All | 34.9 ± 22.0 | 43.8 ± 21.9 | 22.1 ± 14.8 | <0.001 |
| Male | 31.5 ± 18.9 | 39.5 ± 17.8 | 19.9 ± 13.7 | <0.001 | |
| Female | 61.2 ± 28.1 | 78.0 ± 24.0 | 38.8 ± 4.6 | 0.054 | |
| VFMI (cm2/m2) | All | 36.6 ± 21.0 | 44.3 ± 20.5 | 25.5 ± 16.4 | <0.001 |
| Male | 31.5 ± 18.4 | 44.9 ± 20.7 | 26.1 ± 17.2 | <0.001 | |
| Female | 37.2 ± 21.4 | 39.4 ± 20.9 | 21.0 ± 8.2 | 0.217 | |
| ∆L3SMI (cm2/m2/120 days) | −1.70 ± 7.96 | −2.14 ± 4.82 | −1.07 ± 11.13 | 0.609 | |
| ∆SFMI (cm2/m2/120 days) | −0.46 ± 11.34 | 0.35 ± 13.17 | −1.62 ± 8.12 | 0.509 | |
| ∆VFMI (cm2/m2/120 days) | 1.02 ± 12.11 | 0.72 ± 13.96 | 1.44 ± 9.08 | 0.821 | |
| CT examination interval (days) | 127.6 ± 89.5 | 127.0 ± 62.9 | 128.5 ± 119.4 | 0.949 | |
| Administration period of sorafenib (days) | 455 ± 396 | 544 ± 448 | 325 ± 264 | 0.032 | |
| Therapeutic effect (CR/PR/SD/PD) | 3/34/5/19 | 2/18/2/14 | 1/16/3/5 | 0.368 | |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex (male vs. female) | 0.872 (0.365–2.082) | 0.758 | ||
| Age (years) | 0.980 (0.956–1.006) | 0.127 | ||
| Child-Pugh score (6 vs. 5) | 1.630 (0.850–3.154) | 0.141 | ||
| Stage (III vs. IV) | 1.009 (0.526–1.935) | 0.979 | ||
| Combination therapy (yes vs. no) | 1.007 (0.508–1.995) | 0.984 | ||
| BMI (kg/m2) | 0.954 (0.846–1.075) | 0.440 | ||
| Sarcopenia (yes vs. no) | 2.124 (1.137–3.967) | 0.018 | 2.453 (1.273–4.728) | 0.007 |
| ∆L3SMI (≤−5.73 vs. >−5.73) | 2.560 (1.218–5.377) | 0.013 | 4.010 (1.799–8.938) | <0.001 |
| ∆SFMI (≤−5.33 vs. >−5.33) | 2.771 (1.382–5.559) | 0.004 | 4.109 (1.967–8.584) | <0.001 |
| ∆VFMI (≤−3.95 vs. >−3.95) | 0.733 (0.324–1.657) | 0.456 | ||
| Therapeutic effect (PD vs. CR/PR/SD) | 3.049 (1.563–5.949) | 0.001 | 4.603 (2.188–9.683) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11081206
Imai K, Takai K, Miwa T, Taguchi D, Hanai T, Suetsugu A, Shiraki M, Shimizu M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers. 2019; 11(8):1206. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11081206
Chicago/Turabian StyleImai, Kenji, Koji Takai, Takao Miwa, Daisuke Taguchi, Tatsunori Hanai, Atsushi Suetsugu, Makoto Shiraki, and Masahito Shimizu. 2019. "Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib" Cancers 11, no. 8: 1206. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11081206