Mi-RNA-888-5p Is Involved in S-Adenosylmethionine Antitumor Effects in Laryngeal Squamous Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
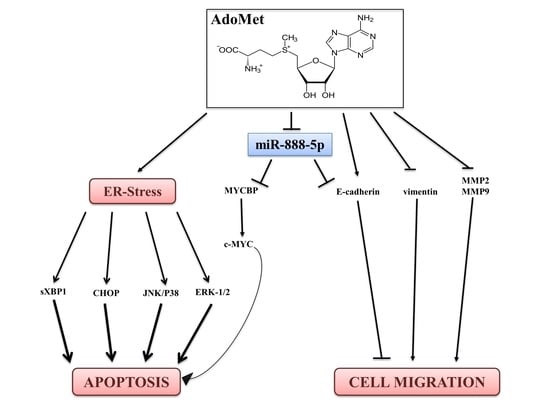
2. Results
2.1. AdoMet Triggered Autophagy in LSCC
2.2. AdoMet Promoted ER-Stress and Related UPR in LSCC
2.3. AdoMet Induced ROS Production in LSCC
2.4. AdoMet Changed miRNA Expression Profile in LSCC
2.5. AdoMet and miR-888-5p Inhibitor Enhanced the Pro-Apoptotic Effect of AdoMet in LSCC
2.6. AdoMet and miR-888-5p Inhibitor Affect EMT in LSCC
2.7. AdoMet Regulates the Expression of MYCBP and CDH1 by the Downregulation of miR-888-5p
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures and Treatments
4.3. LysoTracker-Red Staining
4.4. Determination of ROS by DCF-DA Assay
4.5. ER Tracker Blue-White DPX Staining
4.6. Cell Transfections
4.7. MiRNA Detection and Validation by qRT-PCR
4.8. Flow Cytometry Analysis of Apoptosis
4.9. RNA Isolation, Reverse Transcription, and qRT-PCR
4.10. Migration Process Evaluated by Scratch-Wound Assay
4.11. Protein Extraction and Western Blot Analysis
4.12. TCGAbiolinks Package
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chin, D.; Boyle, G.M.; Porceddu, S.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Head and neck cancer: Past, present and future. Expert Rev. Anticancer Ther. 2006, 6, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Mastronikolis, N.S.; Papadas, T.A.; Goumas, P.D.; Triantaphyllidou, I.E.; Theocharis, D.A.; Papageorgakopoulou, N.; Vynios, D.H. Head and neck: Laryngeal tumors: An overview. Atlas Genet. Cytogenet. Oncol. Haematol. 2009, 13, 888–893. [Google Scholar] [CrossRef]
- Maasland, D.H.E.; Van den Brandt, P.A.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands cohort study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, D.; Oridate, N. The molecular mechanisms of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2016, 21, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakostas, G.I.; Cassiello, C.F.; Iovieno, N. Folates and S-adenosylmethionine for major depressive disorder. Can. J. Psychiatry 2012, 57, 406–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeken, K.L.; Lee, W.L.; Bausell, R.B.; Agelli, M.; Berman, B.M. Safety and efficacy of S-adenosylmethionine (SAMe) for osteoarthritis. J. Fam. Pract. 2002, 51, 425–430. [Google Scholar]
- Li, T.W.; Zhang, Q.; Oh, P.; Xia, M.; Chen, H.; Bemanian, S.; Lastra, N.; Circ, M.; Moyer, M.P.; Mato, J.M.; et al. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol. Pharmacol. 2009, 76, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Li, Y.N.; Wang, F.; Zhang, W.M.; Geng, X. S-adenosylmethionine inhibits the growth of cancer cells by reversing the hypomethylation status of c-Myc and H-ras in human gastric cancer and colon cancer. Int. J. Biol. Sci. 2010, 6, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Ilisso, C.P.; Castellano, M.; Zappavigna, S.; Lombardi, A.; Vitale, G.; Dicitore, A.; Cacciapuoti, G.; Caraglia, M.; Porcelli, M. The methyl donor S-adenosylmethionine potentiates doxorubicin effects on apoptosis of hormone-dependent breast cancer cell lines. Endocrine 2015, 50, 212–222. [Google Scholar] [CrossRef]
- Ilisso, C.P.; Sapio, L.; Delle Cave, D.; Illiano, M.; Spina, A.; Cacciapuoti, G.; Naviglio, S.; Porcelli, M. S-Adenosylmethionine affects ERK1/2 and Stat3 pathways and induces apoptosis in osteosarcoma cells. J. Cell. Physiol. 2016, 231, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, J.; Yang, Q.; Si, Y.; Liu, Y.; Wang, Q.; Han, F.; Huang, Z. Synergistic effects of SAM and selenium compounds on proliferation, migration and adhesion of HeLa cells. Anticancer Res. 2017, 37, 4433–4441. [Google Scholar] [PubMed] [Green Version]
- Cave, D.D.; Ilisso, C.P.; Mosca, L.; Pagano, M.; Martino, E.; Porcelli, M.; Cacciapuoti, G. The anticancer effects of S-Adenosylmethionine on breast cancer cells. JSM Chem. 2017, 5, 1049. [Google Scholar]
- Mahmood, N.; Cheishvili, D.; Arakelian, A.; Tanvir, I.; Khan, H.A.; Pépin, A.S.; Szyf, M.; Rabbani, S.A. Methyl donor S-adenosylmethionine (SAM) supplementation attenuates breast cancer growth, invasion, and metastasis in vivo; therapeutic and chemopreventive applications. Oncotarget 2018, 9, 5169–5183. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.; Pagano, M.; Ilisso, C.P.; Cave, D.D.; Desiderio, V.; Mele, L.; Caraglia, M.; Cacciapuoti, G.; Porcelli, M. AdoMet triggers apoptosis in head and neck squamous cancer by inducing ER stress and potentiates cell sensitivity to cisplatin. J. Cell. Physiol. 2019, 234, 13277–13291. [Google Scholar] [CrossRef]
- Mosca, L.; Minopoli, M.; Pagano, M.; Vitiello, F.; Carriero, M.V.; Cacciapuoti, G.; Porcelli, M. Effects of S-adenosyl-L-methionine on the invasion and migration of head and neck squamous cancer cells and analysis of the underlying mechanisms. Int. J. Oncol. 2020, 56, 1212–1224. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Tingting, B.; Linxun, L.; Quangen, G.; Genhai, S.; Lei, Q. S-Adenosylmethionine synergistically enhances the antitumor effect of gemcitabine against pancreatic cancer through JAK2/STAT3 pathway. Naunyn-schmiedeberg Arch. Pharmacol. 2019, 392, 615–622. [Google Scholar]
- Delle Cave, D.; Desiderio, V.; Mosca, L.; Ilisso, C.P.; Mele, L.; Caraglia, M.; Cacciapuoti, G.; Porcelli, M. S-Adenosylmethionine-mediated apoptosis is potentiated by autophagy inhibition induced by chloroquine in human breast cancer cells. J. Cell. Physiol. 2018, 233, 1370–1383. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-coding RNAs as important biomarkers in laryngeal cancer and other head and neck tumours. Int. J. Mol. Sci. 2019, 20, 3444. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, M.L.; Cossu, C.; Spissu, Y.; Floris, A.; Ryoo1, M.; Iglesias-Ara, A.; Wang, Q.; Pandol, S.J.; Bhowmick, N.A.; Seki, E.; et al. S-adenosylmethionine and methylthioadenosine inhibit cancer metastasis by targeting MicroRNA 34a/b-methionine adenosyltransferase 2A/2B axis. Oncotarget 2017, 8, 78851–78869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilisso, C.P.; Delle Cave, D.; Mosca, L.; Pagano, M.; Coppola, A.; Mele, L.; Caraglia, M.; Cacciapuoti, G.; Porcelli, M. S-Adenosylmethionine regulates apoptosis and autophagy in MCF-7 breast cancer cells through the modulation of specific microRNAs. Cancer Cell Int. 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, M.; Porto, S.; Zappavigna, S.; Stiuso, P.; Tirino, V.; Desiderio, V.; Mele, L.; Caraglia, M. Levofolene modulates apoptosis induced by 5-fluorouracil through autophagy inhibition: Clinical and occupational implications. Int. J. Oncol. 2015, 46, 1893–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmicreticulum stress, UnfoldedProteinResponse, and cancercell fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. ActaBiochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Darling, N.; Cook, S.J. The role of MAPK signaling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Adhikary, A.; Chakraborty, S.; Bhattacharjee, P.; Mazumder, M.; Putatunda, S.; Gorain, M.; Chakraborty, A.; Kundu, G.C.; Das, T.; et al. Cross-talk between endoplasmic reticulum (ER) stress and the MEK/ERK pathway potentiates apoptosis in human triple negative breast carcinoma cells. J. Biol. Chem. 2015, 290, 3936–3949. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, M.; Zheng, Y.; Zhou, L.; Wang, Q.; Chen, L. miR-888 in MCF-7 side population sphere cells directly targets E-cadherin. J. Genet. Genom. 2014, 41, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, W.; Wen, S.; Wu, Y.; Fu, R.; Zhao, D.; Chen, X.; Wang, B. Potential key molecular correlations in laryngeal squamous cell carcinoma revealed by integrated analysis of mRNA, miRNA and lncRNA microarray profiles. Neoplasma 2016, 63, 888–900. [Google Scholar] [CrossRef] [Green Version]
- Zubor, P.; Kubatka, P.; Dankova, Z.; Gondova, A.; Kajo, K.; Hatok, J.; Samec, M.; Jagelkova, M.; Krivus, S.; Holubekova, V.; et al. miRNA in a multiomic context for diagnosis, treatment monitoring and personalized management of metastatic breast cancer. Future Oncol. 2018, 14, 1847–1867. [Google Scholar] [CrossRef]
- Li, G.; Fang, J.; Wang, Y.; Wang, H.; Sun, C.C. MiRNA-based therapeutic strategy in lung cancer. Curr. Pharm. Des. 2018, 23, 6011–6018. [Google Scholar] [CrossRef]
- Vanacore, D.; Boccellino, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Di Franco, R.; Romano, F.J.; Montanari, M.; La Mantia, M.; Piscitelli, R.; et al. Micrornas in prostate cancer: An overview. Oncotarget 2017, 8, 50240–50251. [Google Scholar] [CrossRef] [Green Version]
- Masood, Y.; Kqueen, C.Y.; Rajadurai, P. Role of miRNA in head and neck squamous cell carcinoma. Expert Rev. Anticancer Ther. 2015, 15, 183–197. [Google Scholar] [CrossRef]
- Fu, H.Y.; Okada, K.; Liao, Y.; Tsukamoto, O.; Isomura, T.; Asai, M.; Minamino, T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 2010, 122, 361–369. [Google Scholar] [CrossRef]
- Ventura, J.J.; Cogswell, P.; Flavell, R.A.; Baldwin, A.S., Jr.; Davis, R.J. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004, 18, 2905–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. The effects of induced production of reactive oxygen species in organelles on endoplasmic reticulum stress and on the unfolded protein response in Arabidopsis. Ann. Bot. 2015, 116, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, H.; Lance, R.; Troyer, D.; Beydoun, H.; Hadley, M.; Orians, J.; Benzine, T.; Madric, K.; Semmes, O.J.; Drake, R.; et al. miR-888 is an expressed prostatic secretions-derived microRNA that promotes prostate cell growth and migration. Cell Cycle 2014, 13, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.J.; Chen, L.; Lu, W.; Zhang, L.; Wang, L.; Zhu, H.H. miR-888 functions as an oncogene and predicts poor prognosis in colorectal cancer. Oncol. Lett. 2018, 15, 9101–9109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Chen, L. MiR-888 regulates side population properties and cancer metastasis in breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 450, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Sun, F.N.; Ma, X.Y.; Qu, H.; Yu, Y. MiR-888 promotes cell migration and invasion of hepatocellular carcinoma by targeting SMAD4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2020–2027. [Google Scholar] [PubMed]
- Cao, J.X. miR-888 regulates cancer progression by targeting multiple targets in lung adenocarcinoma. Oncol. Rep. 2019, 41, 3367–3376. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G.; Semb, H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 1999, 24, 73–76. [Google Scholar] [CrossRef]
- Alfano, D.; Votta, G.; Schulze, A.; Downward, J.; Caputi, M.; Stoppelli, M.P.; Iaccarino, I. Modulation of cellular migration and survival by c-Myc through the downregulation of urokinase (uPA) and uPA receptor. Mol. Cell. Biol. 2010, 30, 1838–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerquetti, L.; Sampaoli, C.; De Salvo, M.; Bucci, B.; Argese, N.; Chimento, A.; Vottari, S.; Marchese, R.; Pezzi, V.; Toscano, V.; et al. C-MYC modulation induces responsiveness to paclitaxel in adrenocortical cancer cell lines. Int. J. Oncol. 2015, 46, 2231–2240. [Google Scholar] [CrossRef] [Green Version]
- Babcock, J.T.; Nguyen, H.B.; He, Y.; Hendricks, J.W.; Wek, R.C.; Quilliam, L.A. Mammalian target of rapamycin complex 1 (mTORC1) enhances bortezomib-induced death in tuberous sclerosis complex (TSC)-null cells by a c-MYC-dependent induction of the unfolded protein response. J. Biol. Chem. 2013, 288, 15687–15698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikte, S.; Panchal, N.; Warnes, G. Use of LysoTracker dyes: A flow cytometric study of autophagy. Cytometry A 2014, 85, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef] [PubMed]






| miRNAs | Fold-Changes (Log2) | |||
|---|---|---|---|---|
| JHU-SCC-011 | HNO210 | |||
| Microarray | qRT-PCR | Microarray | qRT-PCR | |
| hsa-miR-888-5p | −2.65 | −7.63 | Not performed | −1.98 |
| hsa-miR-187 | 3.80 | 0.08 | Not performed | 0.01 |
| hsa-miR-491-3p | 5.62 | 0.06 | Not performed | −0.12 |
| hsa-miR-618 | −4.8 | −0.67 | Not performed | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, M.; Mosca, L.; Vitiello, F.; Ilisso, C.P.; Coppola, A.; Borzacchiello, L.; Mele, L.; Caruso, F.P.; Ceccarelli, M.; Caraglia, M.; et al. Mi-RNA-888-5p Is Involved in S-Adenosylmethionine Antitumor Effects in Laryngeal Squamous Cancer Cells. Cancers 2020, 12, 3665. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123665
Pagano M, Mosca L, Vitiello F, Ilisso CP, Coppola A, Borzacchiello L, Mele L, Caruso FP, Ceccarelli M, Caraglia M, et al. Mi-RNA-888-5p Is Involved in S-Adenosylmethionine Antitumor Effects in Laryngeal Squamous Cancer Cells. Cancers. 2020; 12(12):3665. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123665
Chicago/Turabian StylePagano, Martina, Laura Mosca, Francesca Vitiello, Concetta Paola Ilisso, Alessandra Coppola, Luigi Borzacchiello, Luigi Mele, Francesca Pia Caruso, Michele Ceccarelli, Michele Caraglia, and et al. 2020. "Mi-RNA-888-5p Is Involved in S-Adenosylmethionine Antitumor Effects in Laryngeal Squamous Cancer Cells" Cancers 12, no. 12: 3665. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123665