E-Cigarette Exposure Decreases Bone Marrow Hematopoietic Progenitor Cells
Abstract
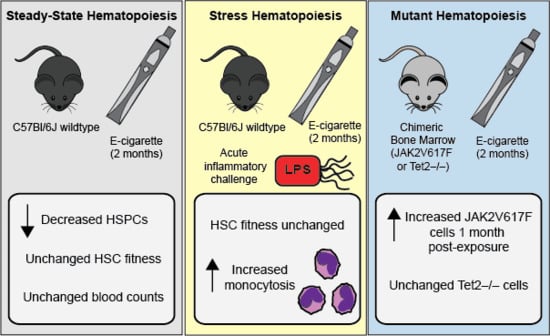
:1. Introduction
2. Results
2.1. E-Cigarette Exposure Does Not Impact Peripheral Blood Counts, Bone Marrow Cellularity, or Mature Cells in the Bone Marrow
2.2. E-Cigarette Exposure Suppresses Myeloid Progenitor Populations
2.3. E-Cigarette Exposure Decreases the Number of Bone Marrow HSPCs
2.4. HSC Function is Unperturbed Following E-Cigarette Smoke Exposure
2.5. E-Cigarette Exposure Does Not Impair HSC Engraftment in Response to LPS
2.6. JAK2V617F Mutant Cells Gain an Early Selective Advantage Following E-Cigarette Exposure
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. E-Cigarette Exposure in Mice
4.3. Peripheral Blood Cell Counts
4.4. Flow Cytometry of Bone Marrow Cell Populations
4.5. Competitive Bone Marrow Transplant and Peripheral Blood Chimerism
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirbolouk, M.; Charkhchi, P.; Kianoush, S.; Uddin, S.M.I.; Orimoloye, O.A.; Jaber, R.; Bhatnagar, A.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; et al. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann. Intern. Med. 2018, 169, 429–438. [Google Scholar] [CrossRef]
- Cullen, K.A.; Gentzke, A.S.; Sawdey, M.D.; Chang, J.T.; Anic, G.M.; Wang, T.W.; Creamer, M.R.; Jamal, A.; Ambrose, B.K.; King, B.A. e-Cigarette Use Among Youth in the United States, 2019. JAMA 2019, 322, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. E-Cigarette Use among Youth and Young Adults: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2016.
- National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of e-Cigarettes; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Orkin, H.S.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, G.M.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Robinson, D.; Allaway, S.L.; Hale, A.C. The effects of cigarette smoking and alcohol consumption on blood haemoglobin, erythrocytes and leucocytes: A dose related study on male subjects. Clin. Lab. Haematol. 1995, 17, 131–138. [Google Scholar] [PubMed]
- Aitchison, R.; Russell, N. Smoking—A major cause of polycythaemia. J. R. Soc. Med. 1988, 81, 89–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Broxmeyer, H.E.; Feng, D.; Schweitzer, K.S.; Yi, R.; Cook, T.G.; Chitteti, B.R.; Barwinska, D.; Traktuev, D.O.; Van Demark, M.J.; et al. Human adipose-derived stem cells ameliorate cigarette smoke-induced murine myelosuppression via secretion of TSG-6. Stem Cells 2015, 33, 468–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselbalch, H.C. Smoking as a contributing factor for development of polycythemia vera and related neoplasms. Leuk Res. 2015, 39, 1137–1145. [Google Scholar] [CrossRef]
- Sørensen, L.A.; Hasselbalch, H.C. Smoking and philadelphia-negative chronic myeloproliferative neoplasms. Eur. J. Haematol. 2016, 97, 63–69. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Bak, M.; Sørensen, A.L.; Zwisler, A.D.; Ellervik, C.; Larsen, M.K.; Hasselbalch, H.C.; Tolstrup, J.S. Smoking is associated with increased risk of myeloproliferative neoplasms: A general population-based cohort study. Cancer Med. 2018, 7, 5796–5802. [Google Scholar] [CrossRef] [Green Version]
- Leal, A.D.; Thompson, C.A.; Wang, A.H.; Vierkant, R.A.; Habermann, T.M.; Ross, J.A.; Mesa, R.A.; Virnig, B.A.; Cerhan, J.R. Anthropometric, medical history and lifestyle risk factors for myeloproliferative neoplasms in the Iowa Women’s Health Study cohort. Int. J. Cancer 2014, 134, 1741–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- James, C.; Ugo, V.; Le Couedic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garcon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Coombs, C.C.; Zehir, A.; Devlin, S.M.; Kishtagari, A.; Syed, A.; Jonsson, P.; Hyman, D.M.; Solit, D.B.; Robson, M.E.; Baselga, J.; et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 2017, 21, 374–382.e4. [Google Scholar] [CrossRef] [Green Version]
- Zink, F.; Stacey, S.N.; Norddahl, G.L.; Frigge, M.L.; Magnusson, O.T.; Jonsdottir, I.; Thorgeirsson, T.E.; Sigurdsson, A.; Gudjonsson, S.A.; Gudmundsson, J.; et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017, 130, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Ebert, B.L. Clonal hematopoiesis in human aging and disease. Science 2019, 366. [Google Scholar] [CrossRef]
- Logue, J.M.; Sleiman, M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Benowitz, N.L.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Assessing Vapers’ Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environ. Sci. Technol. 2017, 51, 9271–9279. [Google Scholar] [CrossRef] [Green Version]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, D.F.; McGown, K.A.; Gallagher, K.; Bengoechea, J.; Dumigan, A.; Einarsson, G.; Elborn, J.S.; Tunney, M.M. Electronic cigarette vapour increases virulence and inflammatory potential of respiratory pathogens. Respir. Res. 2019, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Wiggs, B.; English, D.; Hogg, J.C.; van Eeden, S.F. The effect of cigarette smoking on the bone marrow. Am. J. Respir. Crit. Care Med. 1997, 155, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Rao, D.S.; O’Connell, R.M.; Garcia-Flores, Y.; Baltimore, D. MicroRNA-146a acts as a guardian of the quality and longevity of hematopoietic stem cells in mice. Elife 2013, 2, e00537. [Google Scholar] [CrossRef] [PubMed]
- King, Y.K.; Goodell, M.A. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 2011, 11, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Mehta, A.; de Boer, C.G.; Kowalczyk, M.S.; Lee, K.; Haldeman, P.; Rogel, N.; Knecht, A.R.; Farouq, D.; Regev, A.; et al. Heterogeneous Responses of Hematopoietic Stem Cells to Inflammatory Stimuli Are Altered with Age. Cell Rep. 2018, 25, 2992–3005.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stetka, J.; Vyhlidalova, P.; Lanikova, L.; Koralkova, P.; Gursky, J.; Hlusi, A.; Flodr, P.; Hubackova, S.; Bartek, J.; Hodny, Z.; et al. Addiction to DUSP1 protects JAK2V617F-driven polycythemia vera progenitors against inflammatory stress and DNA damage, allowing chronic proliferation. Oncogene 2019, 38, 5627–5642. [Google Scholar] [CrossRef]
- Cai, Z.; Kotzin, J.J.; Ramdas, B.; Chen, S.; Nelanuthala, S.; Palam, L.R.; Pandey, R.; Mali, R.S.; Liu, Y.; Kelley, M.R.; et al. Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis. Cell Stem Cell 2018, 23, 833–849.e5. [Google Scholar] [CrossRef] [Green Version]
- Khaldoyanidi, S.; Sikora, L.; Orlovskaya, I.; Matrosova, V.; Kozlov, V.; Sriramarao, P. Correlation between nicotine-induced inhibition of hematopoiesis and decreased CD44 expression on bone marrow stromal cells. Blood 2001, 98, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Siggins, R.W.; Hossain, F.; Rehman, T.; Melvan, J.N.; Zhang, P.; Welsh, D.A. Cigarette Smoke Alters the Hematopoietic Stem Cell Niche. Med. Sci. (Basel) 2014, 2, 37–50. [Google Scholar] [CrossRef]
- Barwinska, D.; Oueini, H.; Poirier, C.; Albrecht, M.E.; Bogatcheva, N.V.; Justice, M.J.; Saliba, J.; Schweitzer, K.S.; Broxmeyer, H.E.; March, K.L.; et al. AMD3100 ameliorates cigarette smoke-induced emphysema-like manifestations in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L382–L386. [Google Scholar] [CrossRef]
- Pandit, T.S.; Sikora, L.; Muralidhar, G.; Rao, S.P.; Sriramarao, P. Sustained exposure to nicotine leads to extramedullary hematopoiesis in the spleen. Stem Cells 2006, 24, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Fritsch, K.; Kovtonyuk, L.V.; Saito, Y.; Yakkala, C.; Jacobs, K.; Ahuja, A.K.; Lopes, M.; Hausmann, A.; Hardt, W.D.; et al. Pathogen-Induced TLR4-TRIF Innate Immune Signaling in Hematopoietic Stem Cells Promotes Proliferation but Reduces Competitive Fitness. Cell Stem Cell 2017, 21, 225–240.e5. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; Offner, S.; Blanco-Bose, W.E.; Waibler, Z.; Kalinke, U.; Duchosal, M.A.; Trumpp, A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009, 458, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Mirantes-Barbeito, C.; Fong, S.; Loeffler, D.; Kovtonyuk, L.V.; Zhang, S.; Lakshminarasimhan, R.; Chin, C.P.; Techner, J.M.; Will, B.; et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 2016, 18, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Passegué, E. TNF-α Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 2019, 25, 357–372.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matatall, K.A.; Jeong, M.; Chen, S.; Sun, D.; Chen, F.; Mo, Q.; Kimmel, M.; King, K.Y. Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. Cell Rep. 2016, 17, 2584–2595. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, Y.; Zheng, P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J. Clin. Investig. 2010, 120, 4091–4101. [Google Scholar] [CrossRef] [Green Version]
- Esplin, B.L.; Shimazu, T.; Welner, R.S.; Garrett, K.P.; Nie, L.; Zhang, Q.; Humphrey, M.B.; Yang, Q.; Borghesi, L.A.; Kincade, P.W. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J. Immunol. 2011, 186, 5367–5375. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Wang, Y.; Ding, Y.; Baez, I.; Payne, K.J.; Borghesi, L. Cutting Edge: Hematopoietic Stem Cell Expansion and Common Lymphoid Progenitor Depletion Require Hematopoietic-Derived, Cell-Autonomous TLR4 in a Model of Chronic Endotoxin. J. Immunol. 2015, 195, 2524–2528. [Google Scholar] [CrossRef] [Green Version]
- Beerman, I.; Bhattacharya, D.; Zandi, S.; Sigvardsson, M.; Weissman, I.L.; Bryder, D.; Rossi, D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 5465–5470. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, L.; Foley, G. E-cigarettes and respiratory health: The latest evidence. J. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mullally, A.; Lane, S.W.; Ball, B.; Megerdichian, C.; Okabe, R.; Al-Shahrour, F.; Paktinat, M.; Haydu, J.E.; Housman, E.; Lord, A.M.; et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010, 17, 584–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, D.C.; Shaffer, K.M.; Polizzi, H.; Mascarenhas, J.; Kremyanskaya, M.; Holland, J.; Hoffman, R. Associations of Physical and Psychologic Symptom Burden in Patients With Philadelphia Chromosome-Negative Myeloproliferative Neoplasms. Psychosomatics 2018, 59, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Bolton, K.L.; Ptashkin, R.N.; Gao, T.; Braunstein, L.; Devlin, S.M.; Kelly, D.; Patel, M.; Berthon, A.; Syed, A.; Yabe, M.; et al. Oncologic therapy shapes the fitness landscape of clonal hematopoiesis. bioRxiv 2019, 848739. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.; Bandukwala, H.S.; An, J.; Lamperti, E.D.; Thompson, E.C.; Hastie, R.; Tsangaratou, A.; Rajewsky, K.; Koralov, S.B.; Rao, A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 14566–14571. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, M.T.; Bufalino, C.; Rasmussen, R.; Hyde, D.; Bhalla, D.K.; Mautz, W.J. Toxicity of chemical components of ambient fine particulate matter (PM 2.5) inhaled by aged rats. J. Appl. Toxicol. 2000, 20, 357–364. [Google Scholar] [CrossRef]
- Kleinman, T.M.; Phalen, R.F. Toxicological interactions in the respiratory system after inhalation of ozone and sulfuric acid aerosol mixtures. Inhal. Toxicol. 2006, 18, 295–303. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanathan, G.; Craver-Hoover, B.; Arechavala, R.J.; Herman, D.A.; Chen, J.H.; Lai, H.Y.; Renusch, S.R.; Kleinman, M.T.; Fleischman, A.G. E-Cigarette Exposure Decreases Bone Marrow Hematopoietic Progenitor Cells. Cancers 2020, 12, 2292. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12082292
Ramanathan G, Craver-Hoover B, Arechavala RJ, Herman DA, Chen JH, Lai HY, Renusch SR, Kleinman MT, Fleischman AG. E-Cigarette Exposure Decreases Bone Marrow Hematopoietic Progenitor Cells. Cancers. 2020; 12(8):2292. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12082292
Chicago/Turabian StyleRamanathan, Gajalakshmi, Brianna Craver-Hoover, Rebecca J. Arechavala, David A. Herman, Jane H. Chen, Hew Yeng Lai, Samantha R. Renusch, Michael T. Kleinman, and Angela G. Fleischman. 2020. "E-Cigarette Exposure Decreases Bone Marrow Hematopoietic Progenitor Cells" Cancers 12, no. 8: 2292. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12082292