Cancer-Related Alopecia: From Etiologies to Global Management
Abstract
:Simple Summary
Abstract
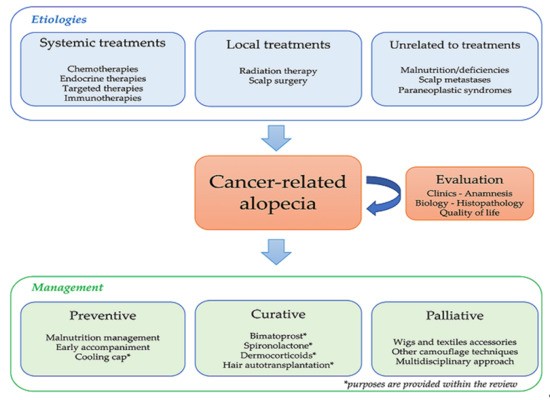
1. Introduction
2. Cancer-Related Alopecia: Mechanisms and Epidemiology
2.1. Classification and Diagnosis
2.2. Chemotherapy
2.3. Focus on Persistent CIA (pCIA)
2.4. Targeted Therapies (TTs)
2.5. Endocrine Therapies (ETs)
2.6. Radiotherapy (RT)
2.7. Immunotherapy
2.8. Other Mechanisms
3. Alopecia Management
3.1. ANIA Prevention
3.2. ANIA Treatments
3.3. Palliative Care and Supplementary Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Blume-Peytavi, U. (Ed.) Hair Growth and Disorders; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-46908-7. [Google Scholar]
- Cho, J.; Choi, E.K.; Kim, I.R.; Im, Y.H.; Park, Y.H.; Lee, S.; Lee, J.E.; Yang, J.H.; Nam, S.J. Development and Validation of Chemotherapy-Induced Alopecia Distress Scale (CADS) for Breast Cancer Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 346–351. [Google Scholar] [CrossRef]
- Macquart-Moulin, G.; Viens, P.; Palangié, T.; Bouscary, M.L.; Delozier, T.; Roché, H.; Janvier, M.; Fabbro, M.; Moatti, J.P. High-Dose Sequential Chemotherapy with Recombinant Granulocyte Colony-Stimulating Factor and Repeated Stem-Cell Support for Inflammatory Breast Cancer Patients: Does Impact on Quality of Life Jeopardize Feasibility and Acceptability of Treatment? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 754–764. [Google Scholar] [CrossRef]
- Carelle, N.; Piotto, E.; Bellanger, A.; Germanaud, J.; Thuillier, A.; Khayat, D. Changing Patient Perceptions of the Side Effects of Cancer Chemotherapy. Cancer 2002, 95, 155–163. [Google Scholar] [CrossRef]
- Mulders, M.; Vingerhoets, A.; Breed, W. The Impact of Cancer and Chemotherapy: Perceptual Similarities and Differences between Cancer Patients, Nurses and Physicians. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2008, 12, 97–102. [Google Scholar] [CrossRef]
- Kang, D.; Kim, I.-R.; Im, Y.H.; Park, Y.H.; Ahn, J.S.; Lee, J.E.; Nam, S.J.; Park, H.; Kim, E.; Lee, H.K.; et al. Quantitative Changes in Skin Composition Parameters Due to Chemotherapy in Breast Cancer Patients: A Cohort Study. Breast Cancer Res. Treat. 2015, 152, 675–682. [Google Scholar] [CrossRef]
- Phillips, G.S.; Freret, M.E.; Friedman, D.N.; Trelles, S.; Kukoyi, O.; Freites-Martinez, A.; Unger, R.H.; Disa, J.J.; Wexler, L.H.; Tinkle, C.L.; et al. Assessment and Treatment Outcomes of Persistent Radiation-Induced Alopecia in Patients with Cancer. JAMA Dermatol. 2020, 156, 963. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Shapiro, J.; Chan, D.; Fornier, M.; Modi, S.; Gajria, D.; Dusza, S.; Goldfarb, S.; Lacouture, M.E. Endocrine Therapy-Induced Alopecia in Patients with Breast Cancer. JAMA Dermatol. 2018, 154, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Saggar, V.; Wu, S.; Dickler, M.N.; Lacouture, M.E. Alopecia with Endocrine Therapies in Patients with Cancer. Oncologist 2013, 18, 1126–1134. [Google Scholar] [CrossRef] [Green Version]
- Freites-Martinez, A.; Shapiro, J.; Goldfarb, S.; Nangia, J.; Jimenez, J.J.; Paus, R.; Lacouture, M.E. Hair Disorders in Patients with Cancer. J. Am. Acad. Dermatol. 2019, 80, 1179–1196. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Shapiro, J.; van den Hurk, C.; Goldfarb, S.; Jimenez, J.J.; Rossi, A.M.; Paus, R.; Lacouture, M.E. Hair Disorders in Cancer Survivors. J. Am. Acad. Dermatol. 2019, 80, 1199–1213. [Google Scholar] [CrossRef]
- Robert, C.; Sibaud, V.; Mateus, C.; Cherpelis, B.S. Advances in the Management of Cutaneous Toxicities of Targeted Therapies. Semin. Oncol. 2012, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Belum, V.R.; Marulanda, K.; Ensslin, C.; Gorcey, L.; Parikh, T.; Wu, S.; Busam, K.J.; Gerber, P.A.; Lacouture, M.E. Alopecia in Patients Treated with Molecularly Targeted Anticancer Therapies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 2496–2502. [Google Scholar] [CrossRef]
- Humbert, P.; Fanian, F.; Maibach, H.I.; Agache, P. Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants; Hair Trichogram; Guichard, A., Fanian, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 801–812. ISBN 978-3-319-32381-7. [Google Scholar]
- Olsen, E.A. Chemotherapy-Induced Alopecia: Overview and Methodology for Characterizing Hair Changes and Regrowth. In The MASCC Textbook of Cancer Supportive Care and Survivorship; Olver, I., Ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-1224-4. [Google Scholar]
- National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 1 June 2021).
- Fischer, T.W.; Schmidt, S.; Strauss, B.; Elsner, P. [Hairdex: A tool for evaluation of disease-specific quality of life in patients with hair diseases]. Der Hautarzt 2001, 52, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bjelic-Radisic, V.; Cardoso, F.; Cameron, D.; Brain, E.; Kuljanic, K.; da Costa, R.A.; Conroy, T.; Inwald, E.C.; Serpentini, S.; Pinto, M.; et al. An International Update of the EORTC Questionnaire for Assessing Quality of Life in Breast Cancer Patients: EORTC QLQ-BR45. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 283–288. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Chan, D.; Sibaud, V.; Shapiro, J.; Fabbrocini, G.; Tosti, A.; Cho, J.; Goldfarb, S.; Modi, S.; Gajria, D.; et al. Assessment of Quality of Life and Treatment Outcomes of Patients with Persistent Postchemotherapy Alopecia. JAMA Dermatol. 2019, 155, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Haslam, I.S.; Sharov, A.A.; Botchkarev, V.A. Pathobiology of Chemotherapy-Induced Hair Loss. Lancet Oncol. 2013, 14, e50–e59. [Google Scholar] [CrossRef]
- Trüeb, R.M. Chemotherapy-Induced Anagen Effluvium: Diffuse or Patterned? Dermatology 2007, 215, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Rzepecki, A.K.; Cheng, H.; McLellan, B.N. Cutaneous Toxicity as a Predictive Biomarker for Clinical Outcome in Patients Receiving Anticancer Therapy. J. Am. Acad. Dermatol. 2018, 79, 545–555. [Google Scholar] [CrossRef]
- Chung, S.; Low, S.-K.; Zembutsu, H.; Takahashi, A.; Kubo, M.; Sasa, M.; Nakamura, Y. A Genome-Wide Association Study of Chemotherapy-Induced Alopecia in Breast Cancer Patients. Breast Cancer Res. BCR 2013, 15, R81. [Google Scholar] [CrossRef] [Green Version]
- Tallon, B.; Blanchard, E.; Goldberg, L.J. Permanent Chemotherapy-Induced Alopecia: Case Report and Review of the Literature. J. Am. Acad. Dermatol. 2010, 63, 333–336. [Google Scholar] [CrossRef]
- Baker, B.W.; Wilson, C.L.; Davis, A.L.; Spearing, R.L.; Hart, D.N.; Heaton, D.C.; Beard, M.E. Busulphan/Cyclophosphamide Conditioning for Bone Marrow Transplantation May Lead to Failure of Hair Regrowth. Bone Marrow Transplant. 1991, 7, 43–47. [Google Scholar]
- Prevezas, C.; Matard, B.; Pinquier, L.; Reygagne, P. Irreversible and Severe Alopecia Following Docetaxel or Paclitaxel Cytotoxic Therapy for Breast Cancer. Br. J. Dermatol. 2009, 160, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, J.R.; Kosche, C.; Choi, J.N. Permanent Chemotherapy-Induced Alopecia: Awareness and Attitudes among Health Care Providers. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 2887–2890. [Google Scholar] [CrossRef]
- Kang, D.; Kim, I.-R.; Choi, E.-K.; Im, Y.H.; Park, Y.H.; Ahn, J.S.; Lee, J.E.; Nam, S.J.; Lee, H.K.; Park, J.-H.; et al. Permanent Chemotherapy-Induced Alopecia in Patients with Breast Cancer: A 3-Year Prospective Cohort Study. Oncologist 2019, 24, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluger, N.; Jacot, W.; Frouin, E.; Rigau, V.; Poujol, S.; Dereure, O.; Guillot, B.; Romieu, G.; Bessis, D. Permanent Scalp Alopecia Related to Breast Cancer Chemotherapy by Sequential Fluorouracil/Epirubicin/Cyclophosphamide (FEC) and Docetaxel: A Prospective Study of 20 Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2879–2884. [Google Scholar] [CrossRef]
- Núñez-Torres, R.; Martín, M.; García-Sáenz, J.Á.; Rodrigo-Faus, M.; del Monte-Millán, M.; Tejera-Pérez, H.; Pita, G.; de la Torre-Montero, J.C.; Pinilla, K.; Herraez, B.; et al. Association Between ABCB1 Genetic Variants and Persistent Chemotherapy-Induced Alopecia in Women with Breast Cancer. JAMA Dermatol. 2020, 156, 987–991. [Google Scholar] [CrossRef]
- Bresters, D.; Wanders, D.C.M.; Louwerens, M.; Ball, L.M.; Fiocco, M.; van Doorn, R. Permanent Diffuse Alopecia after Haematopoietic Stem Cell Transplantation in Childhood. Bone Marrow Transplant. 2017, 52, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Lefrançois, P. Efficacy, Safety, and Comparison of Sonic Hedgehog Inhibitors in Basal Cell Carcinomas: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2018, 79, 1089–1100. [Google Scholar] [CrossRef]
- Alkeraye, S.; Maire, C.; Desmedt, E.; Templier, C.; Mortier, L. Persistent Alopecia Induced by Vismodegib. Br. J. Dermatol. 2015, 172, 1671–1672. [Google Scholar] [CrossRef]
- Peng, C.; Lei, J.-X. The Incidence and Risk of Cutaneous Toxicities Associated with Dabrafenib in Melanoma Patients: A Systematic Review and Meta-Analysis. Eur. J. Hosp. Pharm. Sci. Pract. 2020. [Google Scholar] [CrossRef] [PubMed]
- Agulnik, M.; Yarber, J.L.; Okuno, S.H.; von Mehren, M.; Jovanovic, B.D.; Brockstein, B.E.; Evens, A.M.; Benjamin, R.S. An Open-Label, Multicenter, Phase II Study of Bevacizumab for the Treatment of Angiosarcoma and Epithelioid Hemangioendotheliomas. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 257–263. [Google Scholar] [CrossRef]
- Mir-Bonafé, J.F.; Saceda-Corralo, D.; Vañó-Galván, S. Adverse Hair Reactions to New Targeted Therapies for Cancer. Actas Dermo-Sifiliográficas 2019, 110, 182–192. [Google Scholar] [CrossRef]
- Gallicchio, L.; Calhoun, C.; Helzlsouer, K.J. Aromatase Inhibitor Therapy and Hair Loss among Breast Cancer Survivors. Breast Cancer Res. Treat. 2013, 142, 435–443. [Google Scholar] [CrossRef]
- Puglisi, F.; Aprile, G.; Sobrero, A. Tamoxifen-Induced Total Alopecia. Ann. Intern. Med. 2001, 134, 1154–1155. [Google Scholar] [CrossRef]
- Moscetti, L.; Agnese Fabbri, M.; Sperduti, I.; Fabrizio, N.; Frittelli, P.; Massari, A.; Pompei, L.; D’Auria, G.; Pofi, E.; Ruggeri, E.M. Adjuvant Aromatase Inhibitor Therapy in Early Breast Cancer: What Factors Lead Patients to Discontinue Treatment? Tumori 2015, 101, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Lasheen, S.; Shohdy, K.S.; Kassem, L.; Abdel-Rahman, O. Fatigue, Alopecia and Stomatitis among Patients with Breast Cancer Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors: A Systematic Review and Meta-Analysis. Expert Rev. Anticancer Ther. 2017, 17, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Sonke, G.S.; Hart, L.L.; Campone, M.; Erdkamp, F.; Janni, W.; Verma, S.; Villanueva, C.; Jakobsen, E.; Alba, E.; Wist, E.; et al. Ribociclib with Letrozole vs Letrozole Alone in Elderly Patients with Hormone Receptor-Positive, HER2-Negative Breast Cancer in the Randomized MONALEESA-2 Trial. Breast Cancer Res. Treat. 2018, 167, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Halperin, E.C. Perez & Brady’s Principles and Practice of Radiation Oncology; Wolters Kluwer: Philadelphia, PA, USA, 2019; ISBN 978-1-4963-8679-3. [Google Scholar]
- Lawenda, B.D.; Gagne, H.M.; Gierga, D.P.; Niemierko, A.; Wong, W.M.; Tarbell, N.J.; Chen, G.T.Y.; Hochberg, F.H.; Loeffler, J.S. Permanent Alopecia after Cranial Irradiation: Dose–Response Relationship. Int. J. Radiat. Oncol. 2004, 60, 879–887. [Google Scholar] [CrossRef]
- Min, C.H.; Paganetti, H.; Winey, B.A.; Adams, J.; MacDonald, S.M.; Tarbell, N.J.; Yock, T.I. Evaluation of Permanent Alopecia in Pediatric Medulloblastoma Patients Treated with Proton Radiation. Radiat. Oncol. 2014, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Zarbo, A.; Belum, V.R.; Sibaud, V.; Oudard, S.; Postow, M.A.; Hsieh, J.J.; Motzer, R.J.; Busam, K.J.; Lacouture, M.E. Immune-Related Alopecia (Areata and Universalis) in Cancer Patients Receiving Immune Checkpoint Inhibitors. Br. J. Dermatol. 2017, 176, 1649–1652. [Google Scholar] [CrossRef]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Antoury, L.; Maloney, N.J.; Bach, D.Q.; Goh, C.; Cheng, K. Alopecia Areata as an Immune-Related Adverse Event of Immune Checkpoint Inhibitors: A Review. Dermatol. Ther. 2020, 33, e14171. [Google Scholar] [CrossRef] [PubMed]
- Lakhmiri, M.; Cavelier-Balloy, B.; Lacoste, C.; Cassius, C.; Baroudjian, B.; Delyon, J.; Lebbé, C.; Reygagne, P. Nivolumab-Induced Alopecia Areata: A Reversible Factor of Good Prognosis? JAAD Case Rep. 2018, 4, 761–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Senesse, P.; Bachmann, P.; Bensadoun, R.J.; Besnard, I.; Bourdel-Marchasson, I.; Bouteloup, C.; Crenn, P.; Goldwasser, F.; Guérin, O.; Latino-Martel, P.; et al. Nutrition chez le patient adulte atteint de cancer: Textes courts. Nutrition Clinique et Métabolisme 2012, 26, 151–158. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and Hair Loss: Effects of Nutrient Deficiency and Supplement Use. Dermatol. Pract. Concept. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Kim, M.-J.; Sim, W.-Y.; Lew, B.-L. Alopecia Neoplastica Due to Gastric Adenocarcinoma Metastasis to the Scalp, Presenting as Alopecia: A Case Report and Literature Review. Ann. Dermatol. 2014, 26, 624–627. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, E.; Paraskeva, P.; Smyrnis, A.; Konstantopoulos, K. Alopecia: A Common Paraneoplastic Manifestation of Cholangiocarcinoma in Humans and Animals. Case Rep. 2012, 2012, bcr2012006217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Zhou, G.; Ding, Y.; Zhu, D.; Fang, H. Multiple Paraneoplastic Syndromes: Myasthenia Gravis, Vitiligo, Alopecia Areata, and Oral Lichen Planus Associated with Thymoma. J. Neurol. Sci. 2011, 308, 177–179. [Google Scholar] [CrossRef]
- Čeović, R.; Desnica, L.; Pulanić, D.; Serventi Seiwerth, R.; Ilić, I.; Grce, M.; Mravak Stipetić, M.; Klepac Pulanić, T.; Bilić, E.; Bilić, E.; et al. High Frequency of Cutaneous Manifestations Including Vitiligo and Alopecia Areata in a Prospective Cohort of Patients with Chronic Graft-vs-Host Disease. Croat. Med. J. 2016, 57, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacouture, M.E.; Sibaud, V.; Gerber, P.A.; van den Hurk, C.; Fernández-Peñas, P.; Santini, D.; Jahn, F.; Jordan, K. Prevention and Management of Dermatological Toxicities Related to Anticancer Agents: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 32, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Betticher, D.C.; Delmore, G.; Breitenstein, U.; Anchisi, S.; Zimmerli-Schwab, B.; Müller, A.; von Moos, R.; Hügli-Dayer, A.M.; Schefer, H.; Bodenmann, S.; et al. Efficacy and Tolerability of Two Scalp Cooling Systems for the Prevention of Alopecia Associated with Docetaxel Treatment. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 2565–2573. [Google Scholar] [CrossRef]
- Ross, M.; Fischer-Cartlidge, E. Scalp Cooling: A Literature Review of Efficacy, Safety, and Tolerability for Chemotherapy-Induced Alopecia. Clin. J. Oncol. Nurs. 2017, 21, 226–233. [Google Scholar] [CrossRef]
- Rugo, H.S.; Klein, P.; Melin, S.A.; Hurvitz, S.A.; Melisko, M.E.; Moore, A.; Park, G.; Mitchel, J.; Bågeman, E.; D’Agostino, R.B.; et al. Association Between Use of a Scalp Cooling Device and Alopecia After Chemotherapy for Breast Cancer. JAMA 2017, 317, 606. [Google Scholar] [CrossRef] [Green Version]
- Nangia, J.; Wang, T.; Osborne, C.; Niravath, P.; Otte, K.; Papish, S.; Holmes, F.; Abraham, J.; Lacouture, M.; Courtright, J.; et al. Effect of a Scalp Cooling Device on Alopecia in Women Undergoing Chemotherapy for Breast Cancer: The SCALP Randomized Clinical Trial. JAMA 2017, 317, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Melin, S.A.; Voigt, J. Scalp Cooling with Adjuvant/Neoadjuvant Chemotherapy for Breast Cancer and the Risk of Scalp Metastases: Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2017, 163, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Rugo, H.S.; Voigt, J. Scalp Hypothermia for Preventing Alopecia During Chemotherapy. A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Breast Cancer 2018, 18, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Han, S.; Zhu, Z.; Hu, Y.; Xing, W. Interventions for Preventing Chemotherapy-Induced Alopecia: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Cancer Nurs. 2020. [Google Scholar] [CrossRef]
- Silva, G.D.B.; Ciccolini, K.; Donati, A.; Hurk, C.V.D. Scalp Cooling to Prevent Chemotherapy-Induced Alopecia. An. Bras. Dermatol. 2020, 95, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.; Lemak, N.A.; Valero, V.; Hymes, S.R.; Farmer, K.L.; Hortobagyi, G.N.; Trancik, R.J.; Bandstra, B.A.; Compton, L.D. A Randomized Trial of Minoxidil in Chemotherapy-Induced Alopecia. J. Am. Acad. Dermatol. 1996, 35, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Rozner, R.N.; Freites-Martinez, A.; Shapiro, J.; Geer, E.B.; Goldfarb, S.; Lacouture, M. Safety of 5α-Reductase Inhibitors and Spironolactone in Breast Cancer Patients Receiving Endocrine Therapies. Breast Cancer Res. Treat. 2019, 174, 15–26. [Google Scholar] [CrossRef]
- Barrón-Hernández, Y.L.; Tosti, A. Bimatoprost for the Treatment of Eyelash, Eyebrow and Scalp Alopecia. Expert Opin. Investig. Drugs 2017, 26, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bouhanna, P.; Larif, M.; Guichard, A. Hair Transplantation in Endocrine Therapy–Induced Alopecia. Dermatol. Surg. 2020. publish ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rannan-Eliya, Y.F.; Rannan-Eliya, S.; Graham, K.; Pizer, B.; McDowell, H.P. Surgical Interventions for the Treatment of Radiation-Induced Alopecia in Pediatric Practice. Pediatr. Blood Cancer 2007, 49, 731–736. [Google Scholar] [CrossRef]
- Dunnill, C.J.; Al-Tameemi, W.; Collett, A.; Haslam, I.S.; Georgopoulos, N.T. A Clinical and Biological Guide for Understanding Chemotherapy-Induced Alopecia and Its Prevention. Oncologist 2018, 23, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Clinicaltrials.Gov. Database. Available online: https://clinicaltrials.gov/ct2/results?cond=Cancer+Alopecia&term=&cntry=&state=&city=&dist= (accessed on 1 June 2021).
- Castro, A.R.; Logarinho, E. Tissue Engineering Strategies for Human Hair Follicle Regeneration: How Far from a Hairy Goal? Stem Cells Transl. Med. 2020, 9, 342–350. [Google Scholar] [CrossRef] [Green Version]

| Treatment Type | Clinical Topography | Main Incriminated Mechanism(s) | Time to Onset | Reversibility | Frequency (%) and Range ([]) |
|---|---|---|---|---|---|
| Chemotherapies | Diffuse and +/− total | Cell division blockage and apoptosis Destruction of the follicle | 2–3 weeks from start | Average: 3–6 months post-treatment Irreversible (with certain regimens) | ≈65 [<10–100] |
| Endocrine therapies | Hair thinning AGA-like pattern | Miniaturization of the follicle | 1–91 months | Not systematic | ≈5 [0–25] |
| Targeted therapies | Very variable (target dependent) | Miniaturization of the follicle (+/− destruction) | Very variable | Possible even during treatment Irreversible with some molecules | ≈15 [2–60] |
| Radiotherapy (<43 Gy) | Depending on the radiation field | Destruction of the follicle | 1–3 weeks from start | Average: 2–4 months post-irradiation | ≈75–100 |
| Radiotherapy (≥43 Gy) | Depending on the radiation field | Destruction of the follicle | ≈100 weeks | No (scaring alopecia) | ≈75–100 |
| Immunotherapies | Variable | Cycle blockage and dysimmunity | Variable | Variable | ≈1–2 |
| Clinical Aspect | Absent | Slight | Moderate | Severe | Total | ||
|---|---|---|---|---|---|---|---|
| SALT hair loss (%) | 0 | 1–24 | 25–49 | 50–74 | 75–95 | 96–99 | 100 |
| Olsen grades | 0 | 1 | 2 | 3 | 4a | 4b | 5 |
| CTCAE v5.0 grades | 0 | 1 | 2 | ||||
| Molecule (Class) | All-Grade Estimated Frequency (%) |
|---|---|
| Daunorubicin, doxorubicin, epirubicin (TI2) | ≈80–100 |
| Docetaxel, paclitaxel (taxanes) | |
| High-dose cyclophosphamide (AA) | |
| Etoposide, Idarubicin (TI2) | ≈40–60 |
| Intravenous topotecan, irinotecan (TI1) | |
| Bleomycin (CA) | ≈10–30 |
| Vinblastine, vincristine, vinorelbine (PA) | |
| 5-Fluorouracil, gemcitabine, methotrexate (AM) | |
| Capecitabine (AM) | <10 |
| Carboplatin, cisplatin, oxaliplatin (PS) |
| Molecule (Class) | All-Grade Estimated Frequency (%) |
|---|---|
| SMOi (vismodegib specifically) | 60 |
| Mul-I (e.g., sorafenib, regorafenib) | 25–30 |
| BRAFi (e.g., dabrafenib, vemurafenib) | 20–25 |
| EGFRi (e.g., afatinib, erlotinib) | 5–15 |
| VEGFRi (e.g., axitinib, cabozantinib, pazopanib, sunitinib) | |
| Anti-VEGF (bevacizumab) | |
| Anti-EGFR (e.g., cetuximab) | |
| ALKi (e.g., crizotinib) | |
| MEKi (e.g., trametinib) |
| Molecule (Class) | All-Grade Estimated Frequency (%) |
|---|---|
| All types of endocrine therapies | ≃5% |
| All types of endocrine therapies * | ≃10% |
| Letrozole (AI) + ribociclib (CDK4/6i) | ≃33% |
| Anastrozole (AI) + gosereline (aGnRH) | ≃25% |
| Letrozole (AI) + palbociclib (CDK4/6i) | ≃22% |
| Fulvestrant (AE) + palbociclib (CDK4/6i) | ≃15% |
| Tamoxifen (SERM) then anastrozole (AI) | ≃15% |
| Tamoxifen (SERM) | ≃10% |
| Leuproreline (aGnRH) | ≃10% |
| Exemestane (AI) + aminoglutethimide | ≃10% |
| AI + fulvestrant (AE) | ≃8% |
| Fulvestrant (AE) | ≃2% |
| Anastrozole, letrozole, exemestane (AI) | ≃2% |
| Flutamide, bicalutamide, nilutamide, abiraterone, enzalutamide (ADT) | ≤1% |
| Type of Alopecia | Recommendation(s) | Level of Evidence |
|---|---|---|
| Global approaches |
| |
| CIA |
| II IV |
| pCIA |
| IV IV |
| EIA |
| III IV |
| RIA |
| IV |
| pRIA |
| IV IV |
| IIA |
| IV |
| TIA |
| IV IV |
| Eyebrows/Lashes |
| III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada, S.; Guichard, A.; Fiteni, F. Cancer-Related Alopecia: From Etiologies to Global Management. Cancers 2021, 13, 5556. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13215556
Quesada S, Guichard A, Fiteni F. Cancer-Related Alopecia: From Etiologies to Global Management. Cancers. 2021; 13(21):5556. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13215556
Chicago/Turabian StyleQuesada, Stanislas, Alexandre Guichard, and Frédéric Fiteni. 2021. "Cancer-Related Alopecia: From Etiologies to Global Management" Cancers 13, no. 21: 5556. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13215556