Catalytic Wet Peroxide Oxidation of Cylindrospermopsin over Magnetite in a Continuous Fixed-Bed Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Operating Conditions Study
2.2. Long-Term Stability
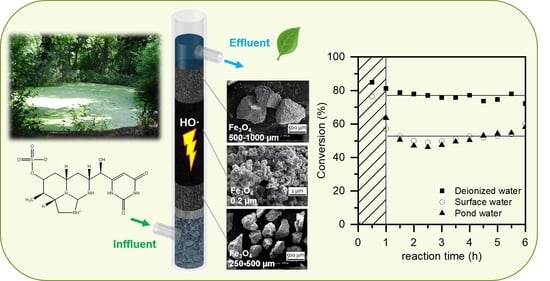
2.3. Operation in Real Water Matrices
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Characterization
3.3. CWPO Experiments
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pantelić, D.; Svirčev, Z.; Simeunović, J.; Vidović, M.; Trajković, I. Cyanotoxins: Characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere 2013, 91, 421–441. [Google Scholar] [CrossRef]
- O’neil, J.; Davis, T.; Burford, M.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Y.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi pour, A. Physico-chemical treatment for the degradation of cyanotoxins with emphasis on drinking water treatment—How far have we come? J. Environ. Chem. Eng. 2018, 6, 5369–5388. [Google Scholar] [CrossRef]
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef]
- Munoz, M.; Nieto-Sandoval, J.; Cirés, S.; de Pedro, Z.M.; Quesada, A.; Casas, J.A. Degradation of widespread cyanotoxins with high impact in drinking water (microcystins, cylindrospermopsin, anatoxin-a and saxitoxin) by CWPO. Water Res. 2019, 163, 114853. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Munoz, M.; Cirés, S.; de Pedro, Z.M.; Colina, J.A.; Velásquez-Figueroa, Y.; Carmona-Jiménez, J.; Caro-Borrero, A.; Salazar, A.; Santa María Fuster, M.C.; Contreras, D.; et al. Overview of toxic cyanobacteria and cyanotoxins in Ibero-American freshwaters: Challenges for risk management and opportunities for removal by advanced technologies. Sci. Total Environ. 2020. under review. [Google Scholar] [CrossRef]
- Wormer, L.; Cirés, S.; Carrasco, D.; Quesada, A. Cylindrospermopsin is not degraded by co-occurring natural bacterial communities during a 40-day study. Harmful Algae 2008, 7, 206–213. [Google Scholar] [CrossRef]
- Armah, A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 2013, 15, 1979–2003. [Google Scholar]
- He, X.; de la Cruz, A.A.; O’Shea, K.E.; Dionysiou, D.D. Kinetics and mechanisms of cylindrospermopsin destruction by sulfate radical-based advanced oxidation processes. Water Res. 2014, 63, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Sievers, M. Advanced oxidation processes. Treatise Water Sci. 2011, 4, 377–408. [Google Scholar]
- Munoz, M.; Pliego, G.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Application of intensified Fenton oxidation to the treatment of sawmill wastewater. Chemosphere 2014, 109, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giméneza, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zeng, J.; Chabi, K.; Song, W.; Xian, X.; Yu, X. Impact of chlorination on cell activation, toxin release and degradation of cyanobacteria of development and maintenance stage. Chem. Eng. J. 2020, 397, 125378. [Google Scholar] [CrossRef]
- Tang, X.; Krausfeldt, L.E.; Shao, K.; LeCleir, G.R.; Stough, J.M.; Gao, G.; Boyer, G.L.; Zhang, Y.; Paerl, H.W.; Qin, B. Seasonal gene expression and the ecophysiological implications of toxic Microcystis aeruginosa blooms in Lake Taihu. Environ. Sci. Technol. 2018, 52, 11049–11059. [Google Scholar] [CrossRef]
- Munoz, M.; Conde, J.; de Pedro, Z.M.; Casas, J.A. Antibiotics abatement in synthetic and real aqueous matrices by H2O2/natural magnetite. Catal. Today 2018, 313, 142–147. [Google Scholar] [CrossRef]
- Serrano, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Efficient removal of the pharmaceutical pollutants included in the EU Watch List (Decision 2015/495) by modified magnetite/H2O2. Chem. Eng. J. 2018, 376, 120265. [Google Scholar] [CrossRef]
- Costa, R.C.C.; Lelis, M.F.F.; Oliveira, L.C.A.; Fabris, J.D.; Ardisson, J.D.; Rios, R.R.V.A.; Silva, C.N.; Lago, R.M. Novel active heterogeneous Fenton system based on Fe3−xMxO4 (Fe, Co, Mn, Ni): The role of M2+ species on the reactivity towards H2O2 reactions. J. Hazard. Mater. 2006, 129, 171–178. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sandell, E.B. Colorimetric Determination of Traces of Metals; Interscience Pubs.: New York, NY, USA, 1959. [Google Scholar]
- Eisenberg, G.M. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. Res. 1943, 15, 327–328. [Google Scholar] [CrossRef]








| Parameter | River Water | Pond Water |
|---|---|---|
| pH | 7.0 | 6.9 |
| TOC (mg L−1) | 2.7 | 4.5 |
| IC (mg L−1) | 14.9 | 47.2 |
| Conductivity (mS cm−1) | 200 | 497 |
| Name | Abbreviation | Molecular Weight | Structural Formula | Reaction |
|---|---|---|---|---|
| Cylindrospermopsin | CYN | 415.4 g mol−1 |  | C15H21N5O7S + 49 H2O2 → 15 CO2 + 56 H2O + 5 HNO3 + H2SO4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; Cirés, S.; M. de Pedro, Z.; Quesada, A.; Casas, J.A. Catalytic Wet Peroxide Oxidation of Cylindrospermopsin over Magnetite in a Continuous Fixed-Bed Reactor. Catalysts 2020, 10, 1250. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111250
Munoz M, Ortiz D, Nieto-Sandoval J, Cirés S, M. de Pedro Z, Quesada A, Casas JA. Catalytic Wet Peroxide Oxidation of Cylindrospermopsin over Magnetite in a Continuous Fixed-Bed Reactor. Catalysts. 2020; 10(11):1250. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111250
Chicago/Turabian StyleMunoz, Macarena, David Ortiz, Julia Nieto-Sandoval, Samuel Cirés, Zahara M. de Pedro, Antonio Quesada, and Jose A. Casas. 2020. "Catalytic Wet Peroxide Oxidation of Cylindrospermopsin over Magnetite in a Continuous Fixed-Bed Reactor" Catalysts 10, no. 11: 1250. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111250