Environmental Assessment of Humic Acid Coated Magnetic Materials Used as Catalyst in Photo-Fenton Processes
Abstract
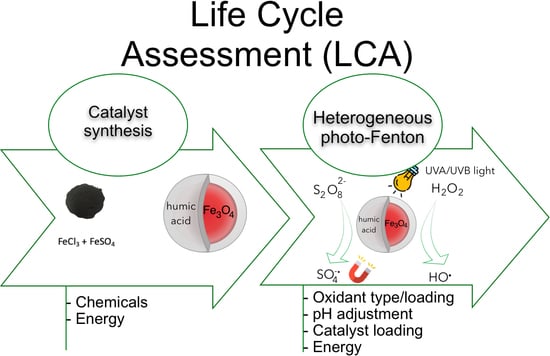
:1. Introduction
2. Results and Discussion
2.1. Life Cycle Impact Assessment of the Catalyst Synthesis
2.2. Life Cycle Impact Assessment (LCIA) of the Degradation Process
2.2.1. Comparative Study between Persulfate and Hydrogen Peroxide
2.2.2. Climate Change Potential
3. Materials and Methods
3.1. Goal and Scope Definition
3.2. Inventory Analysis
3.3. Life Cycle Impact Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications, 1st ed.; IWA Publishing: London, UK, 2018. [Google Scholar]
- World Water Council. Available online: https://www.worldwatercouncil.org/en/water-crisis (accessed on 30 April 2020).
- Liu, J.; Mooney, H.; Hull, V.; Davis, S.J.; Gaskell, J.; Hertel, T.; Lubchenco, J.; Seto, K.C.; Gleick, P.; Kremen, C.; et al. Systems integration for global sustainability. Science 2015, 347, 1258832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davididou, K.; Monteagudo, J.M.; Chatzisymeon, E.; Durán, A.; Expósito, A.J. Degradation and mineralization of antipyrine by UV-A LED photo-Fenton reaction intensified by ferrioxalate with addition of persulfate. Sep. Purif. Technol. 2017, 172, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Balsamo, S.A.; D’Urso, L.; Sciré, S.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Privitera, V.; Impellizzeri, G. CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes. Catalysts 2020, 10, 446. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- García-Ballesteros, S.; Grimalt, J.; Berto, S.; Minella, M.; Laurenti, E.; Vicente, R.; López-Pérez, M.F.; Amat, A.M.; Bianco Prevot, A.; Arques, A. New route for valorization of oil mill wastes: Isolation of humic-like substances to be employed in solar-driven processes for pollutants removal. ACS Omega 2018, 3, 13073–13080. [Google Scholar] [CrossRef] [PubMed]
- Lastre-Acosta, A.M.; Vicente, R.; Mora, M.; Jáuregui-Haza, U.J.; Arques, A.; Teixeira, A.C.S.C. Photo-Fenton reaction at mildly acidic conditions: Assessing the effect of bio-organic substances of different origin and characteristics through experimental design. J. Environ. Sci. Health A 2019, 54, 711–772. [Google Scholar] [CrossRef]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and Photo-Fenton Nanocatalysts Revisited from the Perspective of Life Cycle Assessment. Catalysts 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, N.P.F.; Minella, M.; Mailhot, G.; Brigante, M.; Bianco Prevot, A. Photo-activation of persulfate and hydrogen peroxide by humic acid coated magnetic particles for Bisphenol A degradation. Catal. Today 2019. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Foteinis, S.; Mantzavinos, D.; Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 2013, 54, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Giménez, J.; Bayarri, B.; González, Ó.; Malato, S.; Peral, J.; Esplugas, S. Advanced oxidation processes at laboratory scale: Environmental and economic impacts. ACS Sustain. Chem. Eng. 2015, 3, 3188–3196. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, A.; Rosal, R.; Fernández-Alba, A.R. Life Cycle Assessment of urban wastewater reuse with ozonation as tertiary treatment. Sci. Total Environ. 2009, 407, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental Assessment Solar Photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Montoneri, E.; Bianco Prevot, A.; Avetta, P.; Arques, A.; Carlos, L.; Magnacca, G.; Laurenti, E.; Tabasso, S. Food Wastes Conversion to Products for Use in Chemical and Environmental Technology, Material Science and Agriculture. ChemInform Abstr. 2013, 46. [Google Scholar] [CrossRef]
- Nisticò, R.; Bianco Prevot, A.; Magnacca, G.; Canone, L.; García-Ballesteros, S.; Arques, A. Sustainable magnetic materials (from chitosan and municipal biowaste) for the removal of diclofenac from water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Minella, M.; Fabbri, D.; Calza, P.; Malitesta, C.; Mazzotta, E.; Bianco Prevot, A. Humic Acid Coated Magnetic Particles as Highly Efficient Heterogeneous Photo-Fenton Materials for Wastewater Treatments. Chem. Eng. J. 2020, 390, 124619. [Google Scholar] [CrossRef]
- ISO. Environmental Management—Life Cycle Assessment—Principles and Framework; vol. EN ISO 14040:2006; International Organisation for Standardisation: Brussels, Belgium, 2006. [Google Scholar]
- ISO. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; vol. EN ISO 14044:2006; International Organisation for Standardisation: Brussels, Belgium, 2006. [Google Scholar]
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.G.; Musso, G.; Mendichi, R.; Chierotti, M.R.; Gobetto, R. Biosurfactants from Urban Green Waste. ChemSusChem 2009, 239–247. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recy. 2010, 55, 34–42. [Google Scholar] [CrossRef]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Van Zelm, R. Recipe 2008: A Life Cycle Impact Assessment Method Which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint Level; Ministry of Housing Spatial Planning and the Environment: The Hague, The Netherlands, 2009. [Google Scholar]
- Frischknecht, R.; Editors, N.J.; Althaus, H.; Bauer, C.; Doka, G.; Dones, R.; Hischier, R.; Hellweg, S.; Köllner, T.; Loerincik, Y.; et al. Implementation of Life Cycle Impact Assessment Methods. Ecoinvent Report No. 3, v2.0; Swiss Centre for Life Cycle Inventories: Dübendorf, The Netherlands, 2007. [Google Scholar]
- Bare, J.; Hofstetter, P.; Pennington, D.; Haes, H. Midpoints Versus Endpoints: The Sacrifices and Benefits. Int. J. Life Cycle Ass. 2012, 5, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Blanco, J.; Vidal, A.; Alarcón, D.; Maldonado, M.; Caceres, J.; Gernjak, W. Applied Studies in Solar Photocatalytic Detoxification: An Overview. Sol. Energy 2003, 75, 329–336. [Google Scholar] [CrossRef]



| FeCl3 | FeSO4 | NH3 | N2 | HA | Water | Heat and Stirring | Drying | Centrifugation | Drying | |
|---|---|---|---|---|---|---|---|---|---|---|
| CCP kg CO2 eq | 2.625 | 0.438 | 2.545 | 5.731 | 0.203 | 1.0 × 10−7 | 1.427 | 7.997 | 3.199 | 11.107 |
| ODP kg CFC−11 eq | 1.2 × 10−6 | 3.4 × 10−8 | 5.1× 10−7 | 5.9 × 10−7 | 3.3 × 10−8 | 5.8 × 10−11 | 1.6 × 10−7 | 9.0 × 10−7 | 3.6 × 10−7 | 1.2 × 10−6 |
| TEP kg SO2 eq | 1.4 × 10−2 | 2.5 × 10−3 | 1.5 × 10−2 | 2.4 × 10−2 | 1.9 × 10−3 | 3.0 × 10−6 | 5.6 × 10−3 | 3.1 × 10−2 | 1.3 × 10−2 | 4.4 × 10−2 |
| FEP kg P eq | 1.8 × 10−3 | 3.3 × 10−4 | 2.1 × 10−4 | 4.9 × 10−3 | 8.9 × 10−5 | 2.8 × 10−7 | 4.1 × 10−4 | 2.3 × 10−3 | 9.1 × 10−4 | 3.2 × 10−3 |
| NREU MJ | 29.22 | 5.09 | 43.37 | 66.47 | 3.38 | 0.009 | 18.24 | 102.23 | 40.89 | 141.99 |
| Total | S2O82− | H2SO4 | Fe3O4/HA | NaOH | Electricity | |
|---|---|---|---|---|---|---|
| CCP kg CO2 eq | 0.714 | 3.41 × 10−4 | 7.91 × 10−6 | 3.52 × 10−3 | 4.97 × 10−5 | 7.10 × 10−1 |
| ODP kg CFC−11 eq | 8.02 × 10−8 | 9.19 × 10−11 | 3.17 × 10−12 | 5.03 × 10−10 | 2.98 × 10−11 | 7.96 × 10−8 |
| TEP kg SO2 eq | 0.003 | 2.76 × 10−6 | 3.18 × 10−7 | 1.52 × 10−5 | 2.44 × 10−7 | 2.80 × 10−3 |
| FEP kg P eq | 0.0002 | 1.94 × 10−7 | 9.73 × 10−9 | 1.42 × 10−6 | 2.99 × 10−8 | 2.03 × 10−4 |
| NREU MJ | 9.132 | 5.33 × 10−3 | 3.16 × 10−4 | 4.51 × 10−2 | 5.53 × 10−4 | 9.08 |
| Total | H2O2 | H2SO4 | Fe3O4/HA | NaOH | Electricity | |
|---|---|---|---|---|---|---|
| CCP kg CO2 eq | 7.25 × 10−1 | 4.19 × 10−5 | 7.91 × 10−6 | 3.53 × 10−3 | 4.97 × 10−5 | 7.21 × 10−1 |
| ODP kg CFC−11 eq | 8.14× 10−8 | 3.80 × 10−12 | 3.17 × 10−12 | 5.03 × 10−10 | 2.98 × 10−11 | 8.09 × 10−8 |
| TEP kg SO2 eq | 3.0× 10−3 | 1.69 × 10−7 | 3.18 × 10−7 | 1.52 × 10−5 | 2.44 × 10−7 | 2.85 × 10−3 |
| FEP kg P eq | 2.0 × 10−4 | 1.54 × 10−8 | 9.73 × 10−7 | 1.42 × 10−6 | 2.99 × 10−8 | 2.06 × 10−4 |
| NREU MJ | 9.27 | 6.55 × 10−4 | 3.16 × 10−4 | 4.51 × 10−2 | 5.53 × 10−4 | 9.22 |
| kg CO2 eq | H2O2 Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 0.5 mM | 1 mM | 2 mM | 3 mM | 5 mM | 10 mM | 20 mM | |
| pH 3 | 45.894 | 1.097 | 0.726 | 0.922 | 0.960 | 0.854 | 0.700 | 1.082 |
| pH 4 | 76.487 | 7.652 | 4.176 | 1.643 | 1.534 | 2.090 | 1.002 | 1.062 |
| k (min−1) | H2O2 Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 0.5 mM | 1 mM | 2 mM | 3 mM | 5 mM | 10 mM | 20 mM | |
| pH 3 | 0.0005 | 0.021 | 0.032 | 0.025 | 0.024 | 0.027 | 0.033 | 0.021 |
| pH 4 | 0.0003 | 0.003 | 0.006 | 0.014 | 0.015 | 0.011 | 0.023 | 0.022 |
| CCP kg CO2 eq | Total | H2O2 | H2SO4 | Fe3O4/HA | NaOH | Electricity |
|---|---|---|---|---|---|---|
| [H2O2] = 1 mM | 0.726 | 4.19 × 10−5 | 7.91 × 10−6 | 3.53 × 10−3 | 4.97 × 10−5 | 0.722 |
| [H2O2] = 10 mM | 0.699 | 4.19 × 10−4 | 7.91 × 10−6 | 3.53 × 10−3 | 4.97 × 10−5 | 0.695 |
| Catalyst Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|
| 100 | 200 | 500 | ||||
| k | kg CO2 eq | k | kg CO2 eq | k | kg CO2 eq | |
| pH 3 | 0.0318 | 0.7258 | 0.047 | 0.4967 | 0.077 | 0.3190 |
| pH 4 | 0.0055 | 4.1760 | 0.021 | 1.1010 | 0.010 | 2.3154 |
| pH 6 | 0.0015 | 15.099 | 0.003 | 7.6567 | 0.004 | 5.7571 |
| CCP kg CO2 eq | Total | H2O2 | H2SO4 | Fe3O4/HA | NaOH | Electricity |
|---|---|---|---|---|---|---|
| 100 mg/L | 0.726 | 4.19 × 10−5 | 7.91 × 10−6 | 3.53 × 10−3 | 4.97 × 10−5 | 0.722 |
| 500 mg/L | 0.316 | 4.19 × 10−5 | 7.91 × 10−6 | 1.76 × 10−2 | 4.97 × 10−5 | 0.298 |
| Input | Amount | Unit of Measure | Type of Measure |
|---|---|---|---|
| Transport | 0.075 | tkm | directly measured |
| NaOH | 39.997 | g | estimated value 1 |
| Na4P2O7 | 265.9 | g | directly measured |
| Energy for heating and stirring | 5140 | kJ | estimated value 1 |
| Energy for centrifugation | 0.166 | kWh | directly measured |
| Distilled water | 60 | L | directly measured |
| H2SO4 | 1390 | g | directly measured |
| Energy for centrifugation | 0.166 | kWh | directly measured |
| Distilled water | 30 | L | directly measured |
| Energy to dry | 2880 | kJ | estimated value 1 |
| Input | Amount | Unit of Measure | Type of Measure |
|---|---|---|---|
| FeCl3 | 2581.366 | g | directly measured |
| FeSO4 | 1678.950 | g | directly measured |
| Distilled water | 106.336 | L | directly measured |
| Energy for heating and stirring | 10,312.590 | kJ | estimated value 1 |
| Humic acid 2 | 100.317 | g | directly measured |
| NH3 | 882.789 | g | directly measured |
| Energy to dry | 57,782.590 | kJ | estimated value 1 |
| Energy for centrifugation | 6.420 | kWh | directly measured |
| Energy to dry | 80,253.60 | kJ | estimated value 1 |
| N2 | 21,467.840 | g | directly measured |
| Oxidant Type: | S2O82− | H2O2 | |||
|---|---|---|---|---|---|
| pH 3 | Oxidant (g) | 0.238 | 0.034 | ||
| Catalyst (g) | 0.100 | 0.100 | |||
| Sulfuric acid (g) | 0.049 | 0.049 | |||
| Sodium hydroxide (g) | 0.040 | 0.040 | |||
| Catalyst type: | Fe3O4/HA | Fe3O4 | Fe3O4/HA | Fe3O4 | |
| Energy consumption (kWh) | 1.425 | 2.151 | 1.448 | 3.154 | |
| pH 4 | Oxidant (g) | 0.238 | 0.034 | ||
| Catalyst (g) | 0.100 | 0.100 | |||
| Sulfuric acid (g) | 0.005 | 0.005 | |||
| Sodium hydroxide (g) | 0.004 | 0.004 | |||
| Oxidant type: | Fe3O4/HA | Fe3O4 | Fe3O4/HA | Fe3O4 | |
| Energy consumption (kWh) | 2.601 | 3.049 | 8.373 | 11.808 | |
| pH 6 | Oxidant (g) | 0.238 | 0.034 | ||
| Catalyst (g) | 0.100 | 0.100 | |||
| Sulfuric acid (g) | 4.9 × 10−5 | 4.9 × 10−5 | |||
| Sodium hydroxide (g) | 4 × 10−5 | 4 × 10−5 | |||
| Catalyst type: | Fe3O4/HA | Fe3O4 | Fe3O4/HA | Fe3O4 | |
| Energy consumption (kWh) | 2.990 | 5.233 | 30.297 | 41.865 | |
| pH 6.5 | Oxidant (g) | 0.238 | |||
| Catalyst (g) | 0.1 | ||||
| Sulfuric acid (g) | 1.96 × 10−5 | ||||
| Sodium hydroxide (g) | 1.6 × 10−5 | ||||
| Catalyst type: | Fe3O4/HA | Fe3O4 | |||
| Energy consumption (kWh) | 6.059 | 7.427 | |||
| Oxidant Concentration | 0 mM | 0.5 mM | 1 mM | 2 mM | 3 mM | 5 mM | 10 mM | 20 mM | |
|---|---|---|---|---|---|---|---|---|---|
| pH 3 | H2O2 (g) | 0 | 0.017 | 0.034 | 0.068 | 0.102 | 0.170 | 0.340 | 0.680 |
| Catalyst (g) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Sulfuric acid (g) | 0.049 | 0.049 | 0.049 | 0.049 | 0.049 | 0.049 | 0.049 | 0.049 | |
| Sodium hydroxide (g) | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | |
| Energy consumption (kWh) | 92.103 | 2.193 | 1.448 | 1.842 | 1.919 | 1.706 | 1.396 | 2.162 | |
| pH 4 | H2O2 (g) | 0 | 0.017 | 0.034 | 0.068 | 0.102 | 0.170 | 0.340 | 0.680 |
| Catalyst (g) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Sulfuric acid (g) | 0.0049 | 0.0049 | 0.0049 | 0.0049 | 0.0049 | 0.0049 | 0.0049 | 0.0049 | |
| Sodium hydroxide (g) | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | |
| Energy consumption (kWh) | 153.506 | 15.351 | 8.373 | 3.289 | 3.070 | 4.187 | 2.002 | 2.122 |
| Catalyst Concentration | 100 mg/L | 200 mg/L | 500 mg/L | |
|---|---|---|---|---|
| pH 3 | H2O2 (g) | 0.034 | 0.034 | 0.034 |
| Catalyst (g) | 0.1 | 0.2 | 0.5 | |
| Sulfuric acid (g) | 0.049 | 0.049 | 0.049 | |
| Sodium hydroxide (g) | 0.04 | 0.04 | 0.04 | |
| Energy consumption (kWh) | 1.448 | 0.980 | 0.598 | |
| pH 4 | H2O2 (g) | 0.034 | 0.034 | 0.034 |
| Catalyst (g) | 0.1 | 0.2 | 0.5 | |
| Sulfuric acid (g) | 0.005 | 0.005 | 0.005 | |
| Sodium hydroxide (g) | 0.004 | 0.004 | 0.004 | |
| Energy consumption (kWh) | 8.373 | 2.193 | 4.605 | |
| pH 6 | H2O2 (g) | 0.034 | 0.034 | 0.034 |
| Catalyst (g) | 0.1 | 0.2 | 0.5 | |
| Sulfuric acid (g) | 4.9 × 10−5 | 4.9 × 10−5 | 4.9 × 10−5 | |
| Sodium hydroxide (g) | 4 × 10−5 | 4 × 10−5 | 4 × 105 | |
| Energy consumption (kWh) | 30.297 | 15.351 | 11.513 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costamagna, M.; Gonçalves, N.P.F.; Bianco Prevot, A. Environmental Assessment of Humic Acid Coated Magnetic Materials Used as Catalyst in Photo-Fenton Processes. Catalysts 2020, 10, 771. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070771
Costamagna M, Gonçalves NPF, Bianco Prevot A. Environmental Assessment of Humic Acid Coated Magnetic Materials Used as Catalyst in Photo-Fenton Processes. Catalysts. 2020; 10(7):771. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070771
Chicago/Turabian StyleCostamagna, Mattia, Nuno P. F. Gonçalves, and Alessandra Bianco Prevot. 2020. "Environmental Assessment of Humic Acid Coated Magnetic Materials Used as Catalyst in Photo-Fenton Processes" Catalysts 10, no. 7: 771. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070771