Degradation of Meropenem by Heterogeneous Photocatalysis Using TiO2/Fiberglass Substrates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the TiO2/Fiberglass Substrates
2.2. Photocatalytic Removal of Meropenem
2.3. Mineralization and Biodegradability Studies
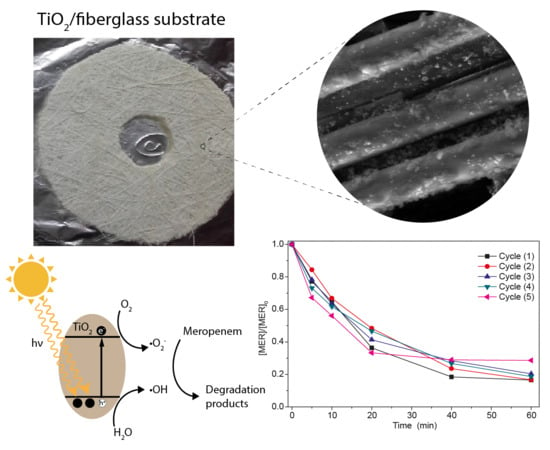
2.4. Reuse and Regeneration of the Material
3. Materials and Methods
3.1. Reagents
3.2. Preparation of TiO2/Fiberglass Substrates
3.3. Photocatalytic Degradation of Meropenem
3.4. Mineralization of MER
3.5. Reuse and Regeneration of the TiO2/Fiberglass Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, R.; Hruby, V. Synthesis of Best-Seller Drugs, 1st ed.; Elsevier: Amsterdam, The Netherland, 2016. [Google Scholar]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Knopp, G.; Dötsch, A.; Wieland, A.; Schwartz, T. Ozone treatment of conditioned wastewater selects antibiotic resistance genes, opportunistic bacteria, and induce strong population shifts. Sci. Total Environ. 2016, 559, 103–112. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Ivekovic, D.; Repec, S.; Stipanicev, D.; Ganjto, M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017, 126, 232–239. [Google Scholar] [CrossRef]
- Szekeres, E.; Baricz, A.; Chiriac, C.M.; Farkas, A.; Opris, O.; Soran, M.-L.; Andrei, A.-S.; Rudi, K.; Balcázar, J.L.; Dragos, N.; et al. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environ. Pollut. 2017, 225, 304–315. [Google Scholar] [CrossRef]
- Kongthavonsakul, K.; Lucksiri, A.; Eakanunkul, S.; Roongjang, S.; Issaranggoon na Ayuthaya, S.; Oberdorfer, P. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int. J. Antimicrob. Agents 2016, 48, 151–157. [Google Scholar] [CrossRef]
- Proia, L.; Anzil, A.; Borrego, C.; Farrè, M.; Llorca, M.; Sanchis, J.; Bogaerts, P.; Balcázar, J.L.; Servais, P. Occurrence and persistence of carbapenemases genes in hospital and wastewater treatment plants and propagation in the receiving river. J. Hazard. Mater. 2018, 358, 33–43. [Google Scholar] [CrossRef]
- Reina, A.C.; Martínez-Piernas, A.B.; Bertakis, Y.; Brebou, C.; Xekoukoulotakis, N.P.; Agüera, A.; Sánchez Pérez, J.A. Photochemical degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions under solar radiation. Water Res. 2018, 128, 61–70. [Google Scholar] [CrossRef]
- Khan, A.; Sharma, D.; Faheem, M.; Bisht, D.; Khan, A.U. Proteomic analysis of a carbapenem-resistant Klebsiella pneumoniae strain in response to meropenem stress. J. Glob. Antimicrob. Resist. 2017, 8, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, M.J.T.L.; Pontes, G.; Serra, P.T.; Balieiro, A.; Castro, D.; Pieri, F.A.; Crainey, J.L.; Nogueira, P.A.; Orlandi, P.P. Multidrug resistant Pseudomonas aeruginosa survey in a stream receiving effluents from ineffective wastewater hospital plants. BMC Microbiol. 2016, 16, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Kordestani, B.; Takdastan, A.; Jalilzadeh Yengejeh, R.; Neisi, A.K. Photo-Fenton oxidative of pharmaceutical wastewater containing meropenem and ceftriaxone antibiotics: Influential factors, feasibility, and biodegradability studies. Toxin Rev. 2018, 1–11. [Google Scholar] [CrossRef]
- Kordestani, B.; Jalilzadeh Yengejeh, R.; Takdastan, A.; Neisi, A.K. A new study on photocatalytic degradation of meropenem and ceftriaxone antibiotics based on sulfate radicals: Influential factors, biodegradability, mineralization approach. Microchem. J. 2019, 146, 286–292. [Google Scholar] [CrossRef]
- Giri, A.S.; Golder, A.K. Fenton, Photo-Fenton, H2O2 Photolysis, and TiO2 Photocatalysis for Dipyrone Oxidation: Drug Removal, Mineralization, Biodegradability, and Degradation Mechanism. Ind. Eng. Chem. Res. 2014, 53, 1351–1358. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.Ν.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Lanceros-Mendez, S.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO 2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Babić, S.; Ćurković, L.; Ljubas, D.; Čizmić, M. TiO2 assisted photocatalytic degradation of macrolide antibiotics. Curr. Opin. Green Sustain. Chem. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Sousa, V.M.; Manaia, C.M.; Mendes, A.; Nunes, O.C. Photoinactivation of various antibiotic resistant strains of Escherichia coli using a paint coat. J. Photochem. Photobiol. A Chem. 2013, 251, 148–153. [Google Scholar] [CrossRef]
- Sandoval, C.; Molina, G.; Vargas Jentzsch, P.; Pérez, J.; Muñoz, F. Photocatalytic Degradation of Azo Dyes Over Semiconductors Supported on Polyethylene Terephthalate and Polystyrene Substrates. J. Adv. Oxid. Technol. 2017, 20. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Ding, Y.; Chen, Y.; Li, P.; Zhu, S.; Shen, S. Highly efficient Zr doped-TiO2/glass fiber photocatalyst and its performance in formaldehyde removal under visible light. J. Environ. Sci. 2017, 60, 61–69. [Google Scholar] [CrossRef]
- Balandina, T.A.; Larina, T.Y.; Kuznetsova, N.I.; Bal’zhinimaev, B.S. Copper catalysts based on fiberglass supports for hydrocarbon oxidation reactions with the participation of hydrogen peroxide. Kinet. Catal. 2008, 49, 499–505. [Google Scholar] [CrossRef]
- Bal’zhinimaev, B.S.; Kovalyov, E.V.; Kaichev, V.V.; Suknev, A.P.; Zaikovskii, V.I. Catalytic Abatement of VOC Over Novel Pt Fiberglass Catalysts. Top. Catal. 2017, 60, 73–82. [Google Scholar] [CrossRef]
- Brichkov, A.S.; Shamsutdinova, A.N.; Khalipova, O.S.; Rogacheva, A.O.; Larina, T.V.; Glazneva, T.S.; Cherepanova, S.V.; Paukshtis, E.A.; Buzaev, A.A.; Kozik, V.V.; et al. Preparation of a fiberglass-supported Ni-Si-Ti oxide catalyst for oxidation of hydrocarbons: Effect of SiO2. J. Chem. Technol. Biotechnol. 2019, 94, 3618–3624. [Google Scholar] [CrossRef]
- Sun, P.; Xue, R.; Zhang, W.; Zada, I.; Liu, Q.; Gu, J.; Su, H.; Zhang, Z.; Zhang, J.; Zhang, D. Photocatalyst of organic pollutants decomposition: TiO2/glass fiber cloth composites. Catal. Today 2016, 274, 2–7. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Liu, C. The Preparation of TiO2 Film Loaded on Fiberglass Mesh and the Experimental Study on Its Photocatalytic Properties. Adv. Mater. Res. 2010, 150, 1421–1424. [Google Scholar] [CrossRef]
- Sriprang, P.; Wongnawa, S.; Sirichote, O. Amorphous titanium dioxide as an adsorbent for dye polluted water and its recyclability. J. Sol-Gel Sci. Technol. 2014, 71, 86–95. [Google Scholar] [CrossRef]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
- Vu, D.; Li, Z.; Zhang, H.; Wang, W.; Wang, Z.; Xu, X.; Dong, B.; Wang, C. Adsorption of Cu(II) from aqueous solution by anatase mesoporous TiO2 nanofibers prepared via electrospinning. J. Colloid Interface Sci. 2012, 367, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Çubuk Demiralay, E.; Koç, D.; Daldal, Y.D.; Alsancak, G.; Ozkan, S.A. Determination of chromatographic dissociation constants of some carbapenem group antibiotics and quantification of these compounds in human urine. Biomed. Chromatogr. 2014, 28, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Picho-Chillán, G.; Dante, R.C.; Muñoz-Bisesti, F.; Martín-Ramos, P.; Chamorro-Posada, P.; Vargas-Jentzsch, P.; Sánchez-Arévalo, F.M.; Sandoval-Pauker, C.; Rutto, D. Photodegradation of Direct Blue 1 azo dye by polymeric carbon nitride irradiated with accelerated electrons. Mater. Chem. Phys. 2019, 237, 121878. [Google Scholar] [CrossRef]
- Parsa, J.B.; Golmirzaei, M.; Abbasi, M. Degradation of azo dye C.I. Acid Red 18 in aqueous solution by ozone-electrolysis process. J. Ind. Eng. Chem. 2014, 20, 689–694. [Google Scholar] [CrossRef]
- Chekem, C.T.; Richardson, Y.; Drobek, M.; Plantard, G.; Blin, J.; Goetz, V. Effective coupling of phenol adsorption and photodegradation at the surface of micro-and mesoporous TiO2-activated carbon materials. React. Kinet. Mech. Catal. 2017, 122, 1297–1321. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Luo, H.; Xu, P. Enhanced photocatalysis using side-glowing optical fibers coated with Fe-doped TiO2 nanocomposite thin films. J. Photochem. Photobiol. A Chem. 2015, 307, 88–98. [Google Scholar] [CrossRef]
- Meng, A.; Xing, J.; Guo, W.; Li, Z.; Wang, X. Electrospinning synthesis of porous Bi12TiO20/Bi4Ti3O12 composite nanofibers and their photocatalytic property under simulated sunlight. J. Mater. Sci. 2018, 53, 14328–14336. [Google Scholar] [CrossRef]
- Wu, H.S.; Wang, Y.L. Inactivation and Regeneration of Photocatalytic Properties of ZnO Nanofilm Materials. Adv. Mater. Res. 2012, 531, 199–202. [Google Scholar] [CrossRef]
- Newcombe, G.; Hayes, R.; Drikas, M. Granular activated carbon: Importance of surface properties in the adsorption of naturally occurring organics. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 78, 65–71. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Rashad, M.; AL-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigments 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]





| pH | k (min−1) | R2 |
|---|---|---|
| 4.0 | 0.032 ± 0.001 | 0.946 ± 0.020 |
| 5.7 | 0.032 ± 0.001 | 0.933 ± 0.009 |
| 7.9 | 0.028 ± 0.001 | 0.976 ± 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamirano Briones, A.; Cóndor Guevara, I.; Mena, D.; Espinoza, I.; Sandoval-Pauker, C.; Ramos Guerrero, L.; Vargas Jentzsch, P.; Muñoz Bisesti, F. Degradation of Meropenem by Heterogeneous Photocatalysis Using TiO2/Fiberglass Substrates. Catalysts 2020, 10, 344. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030344
Altamirano Briones A, Cóndor Guevara I, Mena D, Espinoza I, Sandoval-Pauker C, Ramos Guerrero L, Vargas Jentzsch P, Muñoz Bisesti F. Degradation of Meropenem by Heterogeneous Photocatalysis Using TiO2/Fiberglass Substrates. Catalysts. 2020; 10(3):344. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030344
Chicago/Turabian StyleAltamirano Briones, Alejandro, Iván Cóndor Guevara, David Mena, Isabel Espinoza, Christian Sandoval-Pauker, Luis Ramos Guerrero, Paul Vargas Jentzsch, and Florinella Muñoz Bisesti. 2020. "Degradation of Meropenem by Heterogeneous Photocatalysis Using TiO2/Fiberglass Substrates" Catalysts 10, no. 3: 344. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030344