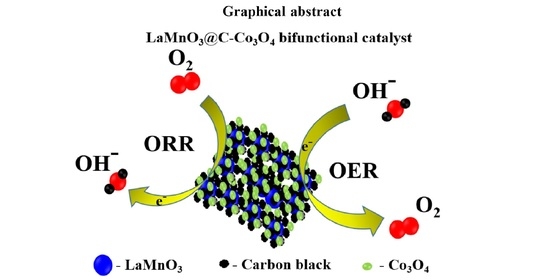
Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Electrocatalytic Activity of the Catalyst
3. Experimental Section
3.1. Materials and Techniques
3.2. Synthesis of LaMnO3 Perovskite Catalyst
3.3. Preparation and Fabrication of LaMnO3@C-Co3O4 Composite Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, L.; Yang, H.; Zhao, S.; Xu, H.; Wu, G. Nanocarbon/oxide composite catalysts for bifunctional oxygen reduction and evolution in reversible alkaline fuel cells: A mini review. J. Power Sources 2018, 375, 277–290. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Q.; Shi, L.; Shi, Z.; Huang, H. Silver decorated LaMnO3 nanorod/graphene composite electrocatalysts as reversible metal-air battery electrodes. Appl. Surf. Sci. 2017, 402, 61–69. [Google Scholar] [CrossRef]
- Oh, M.Y.; Lee, J.J.; Park, H.S.; Kim, T.-Y.; Lee, Y.-S.; Vanchiappan, A.; Nahm, K.S.; Aravindan, V. Efficient bifunctional catalytic activity of nanoscopic Pd-decorated La0.6Sr0.4CoO3-perovskite toward Li–O2 battery, oxygen reduction, and oxygen evolution reactions. J. Ind. Eng. Chem. 2019, 80, 686–695. [Google Scholar] [CrossRef]
- Rani, K.K.; Karuppiah, C.; Wang, S.-F.; Alaswad, S.O.; Sireesha, P.; Devasenathipathy, R.; Jose, R.; Yang, C.-C. Direct pyrolysis and ultrasound assisted preparation of N, S co-doped graphene/Fe3C nanocomposite as an efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Ultrason. Sonochemistry 2020, 66, 105111. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Wang, H.; Cao, Y.; Yu, H.; Peng, F. Co9S8-porous carbon spheres as bifunctional electrocatalysts with high activity and stability for oxygen reduction and evolution reactions. Electrochim. Acta 2018, 265, 32–40. [Google Scholar] [CrossRef]
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional Perovskite Oxide Catalysts for Oxygen Reduction and Evolution in Alkaline Media. Chem. Asian J. 2016, 11, 10–21. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Khraisheh, M.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Wang, Q.; Wang, X.; Qian, D.; Jiang, J.; Li, J.; Chen, Z. Designed synthesis of LaCoO3/N-doped reduced graphene oxide nanohybrid as an efficient bifunctional electrocatalyst for ORR and OER in alkaline medium. J. Alloy. Compd. 2017, 725, 260–269. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Zamani, P.; Seo, M.H.; Nazar, L.F.; Chen, Z. Electrospun porous nanorod perovskite oxide/nitrogen-doped graphene composite as a bi-functional catalyst for metal air batteries. Nano Energy 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Ge, X.; Goh, F.W.T.; Li, B.; Chen, H.W.; Zhang, J.; Xiao, P.; Wang, X.; Zong, Y.; Liu, Z. Efficient and durable oxygen reduction and evolution of a hydrothermally synthesized La(Co0.55Mn0.45)0.99O3−δ nanorod/graphene hybrid in alkaline media. Nanoscale 2015, 7, 9046–9054. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Lin, Y.; Yu, X.; Xu, W.; Salas, T.; Smallidge, H.; Zhou, M.; Luo, H. La0.8Sr0.2MnO3-Based Perovskite Nanoparticles with the A-Site Deficiency as High Performance Bifunctional Oxygen Catalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 23820–23827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, S.; Kim, J.; Cha, S.-W.; Lim, J. Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting. Catalysts 2020, 10, 770. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Z.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Copper/cobalt-doped LaMnO3 perovskite oxide as a bifunctional catalyst for rechargeable Li-O2 batteries. J. Alloy. Compd. 2019, 801, 19–26. [Google Scholar] [CrossRef]
- Karuppiah, C.; Rani, K.K.; Wang, S.-F.; Devasenathipathy, R.; Yang, C.-C. Dry particle coating preparation of highly conductive LaMnO3@C composite for the oxygen reduction reaction and hydrogen peroxide sensing. J. Taiwan Inst. Chem. Eng. 2018, 93, 94–102. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.; Su, C.; Lu, B.; Shao, Z.; Huang, H. Anchoring perovskite LaMnO3 nanoparticles on biomass−derived N, P co−doped porous carbon for efficient oxygen reduction. Electrochim. Acta 2018, 274, 40–48. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, M.G.; Park, H.W.; Seo, M.H.; Ismayilov, V.; Ahmed, R.; Chen, Z. Highly active Co-doped LaMnO 3 perovskite oxide and N-doped carbon nanotube hybrid bi-functional catalyst for rechargeable zinc–air batteries. Electrochem. Commun. 2015, 60, 38–41. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chena, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Ma, P.; Lei, N.; Yu, B.; Liu, Y.K.; Jiang, G.; Dai, J.M.; Li, S.H.; Lu, Q.L. Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition. Nanomaterials 2019, 9, 1676. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yin, F.; Li, Y.; Wang, H.; Chen, J.; Wang, Y.; Chen, B. NiMnO3/NiMn2O4 Oxides Synthesized via the Aid of Pollen: Ilmenite/Spinel Hybrid Nanoparticles for Highly Efficient Bifunctional Oxygen Electrocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 26740–26757. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, S.S.; Tran, D.T.; Read, J. Oxygen reduction reaction catalyst on lithium/air battery discharge performance. J. Mater. Chem. 2011, 21, 10118–10125. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K.; Parvez, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Choi, B.; Kim, Y. Development of Highly Active Bifunctional Electrocatalyst Using Co3O4 on Carbon Nanotubes for Oxygen Reduction and Oxygen Evolution. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; He, C.; Sun, F.; Ding, Y.; Wang, M.; Peng, L.; Wang, J.; Lin, Y. Co3O4 nanoparticles anchored on nitrogen-doped reduced graphene oxide as a multifunctional catalyst for H2O2 reduction, oxygen reduction and evolution reaction. Sci. Rep. 2017, 7, srep43638. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, T.; Gong, H.; Guo, H.; Fan, X.; Song, L.; Xia, W.; Feng, Y.; Hea, J. Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction. Catalysts 2017, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, Y.; Ciucci, F. Mechanochemical Coupling of MoS2 and Perovskites for Hydrogen Generation. ACS Appl. Energy Mater. 2018, 1, 6409–6416. [Google Scholar] [CrossRef]
- Curcio, A.; Wang, J.; Wang, Z.; Zhang, Z.; Belotti, A.; Pepe, S.; Effat, M.B.; Shao, Z.; Lim, J.; Ciucci, F. Unlocking the Potential of Mechanochemical Coupling: Boosting the Oxygen Evolution Reaction by Mating Proton Acceptors with Electron Donors. Adv. Funct. Mater. 2020, 2008077, 1–14. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Baer, D.R.; Herrera-Gómez, A.; Sherwood, P.M.A. Introductory guide to backgrounds in XPS spectra and their impact on determining peak intensities. J. Vac. Sci. Technol. A 2020, 38, 063203. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Y.; Jin, C.; Li, F.; Yang, R.; Chen, F. Microporous La0.8Sr0.2MnO3 perovskite nanorods as efficient electrocatalysts for lithium–air battery. J. Power Sources 2015, 293, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Jeong, M.-G.; Oh, I.-H.; Nam, K.-W.; Jung, H.-G. Role of strontium as doping agent in LaMn0.5Ni0.5O3 for oxygen electro-catalysis. J. Ind. Eng. Chem. 2020, 85, 94–101. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Vengalis, B.; Šliužienė, K.; Orlowski, B.A.; Osinniy, V.; Drube, W. Investigation of epitaxial LaNiO3−x thin films by high-energy XPS. J. Alloy. Compd. 2006, 423, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Li, J.P.H.; Zhou, X.; Pang, Y.; Zhu, L.; Vovk, E.I.; Cong, L.; Van Bavel, A.P.; Li, S.; Yang, Y. Understanding of binding energy calibration in XPS of lanthanum oxide by in situ treatment. Phys. Chem. Chem. Phys. 2019, 21, 22351–22358. [Google Scholar] [CrossRef] [PubMed]
- Bin Yousaf, A.; Imran, M.; Farooq, M.; Kasak, P. Interfacial Phenomenon and Nanostructural Enhancements in Palladium Loaded Lanthanum Hydroxide Nanorods for Heterogeneous Catalytic Applications. Sci. Rep. 2018, 8, 4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharbi, B.; Boukhachem, A.; Amlouk, M.; Oueslati, M.; Dkhil, B.; Meftah, A. Physical investigations on LaMn1−xNixO3 perovskite sprayed thin films along with surface magnetic applications. Appl. Phys. A 2020, 126, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Lu, F.; Jin, C.; Wu, J.; Shen, M.; Yang, R.; Chen, F. Carbon-coating functionalized La0.6Sr1.4MnO4+δ layered perovskite oxide: Enhanced catalytic activity for the oxygen reduction reaction. RSC Adv. 2014, 5, 974–980. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Gao, Y.; Chen, D.; Chen, C.; Saccoccio, M.; Ciucci, F. Ba0.5Sr0.5Co0.8Fe0.2O3−δ on N-doped mesoporous carbon derived from organic waste as a bi-functional oxygen catalyst. Int. J. Hydrogen Energy 2016, 41, 10744–10754. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Matin, A.; Tarlochan, F. Synthesis of Highly Efficient Bifunctional Ag/Co3O4 Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Medium. ACS Omega 2018, 3, 7745–7756. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chen, X.; Evans, D.G.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed hard carbon spherules with uniform nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppiah, C.; Thirumalraj, B.; Alagar, S.; Piraman, S.; Li, Y.-J.J.; Yang, C.-C. Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis. Catalysts 2021, 11, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010076
Karuppiah C, Thirumalraj B, Alagar S, Piraman S, Li Y-JJ, Yang C-C. Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis. Catalysts. 2021; 11(1):76. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010076
Chicago/Turabian StyleKaruppiah, Chelladurai, Balamurugan Thirumalraj, Srinivasan Alagar, Shakkthivel Piraman, Ying-Jeng Jame Li, and Chun-Chen Yang. 2021. "Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis" Catalysts 11, no. 1: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010076