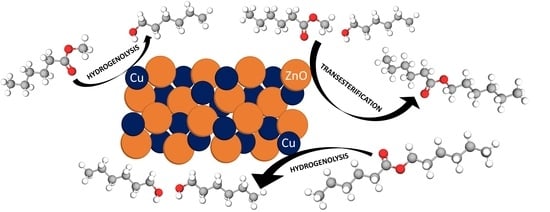
Understanding of the Key Factors Determining the Activity and Selectivity of CuZn Catalysts in Hydrogenolysis of Alkyl Esters to Alcohols
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Characterization
2.1.1. Chemical Analysis
2.1.2. XRD Study
2.1.3. Catalyst Surface Area
2.1.4. TPD of Adsorbed CO2 and NH3
2.1.5. Specific Copper Surface Area
2.2. Catalysis
2.2.1. The Hydrogenolysis of Methyl Hexanoate (MeHe) in Presence of CuZn-R Catalysts Varied in Cu/Zn Ratio
2.2.2. The Hydrogenolysis of Hexyl Hexanoate in Presence of CuZn-R Catalysts with Different Cu/Zn Ratio
2.2.3. The Stability of CuZn Catalysts in Repeating Reaction Cycles
2.2.4. The Hydrogenolysis of Methyl Esters with the Different Length of Carbon Chain
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Catalyst Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turek, T.; Trimm, D.L.; Cant, N.W. The catalytic hydrogenolysis of esters to alcohols. Catal. Rev. Sci. Eng. 1994, 36, 645–683. [Google Scholar] [CrossRef]
- Myers, M. Surfactant Science and Technology; John Wiley: New York, NY, USA, 2006. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Adkins, H.; Folkers, K. The catalytic hydrogenolysis of esters to alcohols. J. Am. Chem. Soc. 1931, 53, 1095–1097. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-M.; Shi, L. Zn promoted Cu–Al catalyst for hydrogenation of ethyl acetate to alcohol. J. Ind. Eng. Chem. 2014, 20, 2341–2347. [Google Scholar] [CrossRef]
- Hu, Q.; Fan, G.; Yang, L.; Li, F. Aluminum-Doped Zirconia-Supported Copper Nanocatalysts: Surface Synergistic Catalytic Effects in the Gas-Phase Hydrogenation of Esters. ChemCatChem 2014, 6, 3501–3510. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, H.; Zhao, Y.; Chen, S.; Liu, Z. Diatomite-supported Pd–M (M=Cu, Co, Ni) bimetal nanocatalysts for selective hydrogenation of long-chain aliphatic esters. J. Colloid Interface Sci. 2012, 386, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadenas, A.F.; Kapteijn, F.; Zieverink, M.M.P.; Moulijn, J.A. Selective hydrogenation of fatty acid methyl esters over palladium on carbon-based monoliths: Structural control of activity and selectivity. Catal. Today 2007, 128, 13–17. [Google Scholar] [CrossRef]
- Figueiredo, F.C.A.; Jordăo, E.; Carvalho, W.A. Adipic ester hydrogenation catalyzed by platinum supported in alumina, titania and pillared clays. Appl. Catal. A General 2008, 351, 259–266. [Google Scholar] [CrossRef]
- Pritchard, J.; Ciftci, A.; Verhoeven, M.W.G.M.; Hensen, E.J.M.; Pidko, E.A. Supported Pt-Re catalysts for the selective hydrogenation of methyl and ethyl esters to alcohols. Catal. Today 2017, 279, 10–18. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Marín, J.M.; Restrepo, G.M.; Riso, L.A. Characterization and carbonylic hydrogenation of methyl oleate over Ru-Sn/Al2O3: Effects of metal precursor and chlorine removal. Appl. Catal. A General 2009, 366, 342–347. [Google Scholar] [CrossRef]
- Luo, G.; Yan, S.; Qiao, M.; Zhuang, J.; Fan, K. Effect of tin on Ru-B/c-Al2O3 catalyst for the hydrogenation of ethyl lactate to 1,2-propanediol. Appl. Catal. A General 2004, 275, 95–102. [Google Scholar] [CrossRef]
- Miyake, T.; Makino, T.; Taniguchi, S.; Watanuki, H.; Niki, T.; Shimizu, S.; Kojima, Y.; Sano, M. Alcohol synthesis by hydrogenation of fatty acid methyl esters on supported Ru–Sn and Rh–Sn catalysts. Appl. Catal. A General 2009, 364, 108–112. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wang, F.; Deng, L.; Ren, J.; Jiao, Z.; Zhou, G. Effect of surface composition and structure of the mesoporous Ni/KIT-6 catalyst on catalytic hydrodeoxygenation performance. Catalysts 2019, 9, 889. [Google Scholar] [CrossRef] [Green Version]
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts. Appl. Catal. A General 1999, 184, 279–289. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Wang, S.; Cao, G. Deactivation Mechanism of Cu/Zn Catalyst Poisoned by Organic Chlorides in Hydrogenation of Fatty Methyl Ester to Fatty Alcohol. Catal. Lett. 2010, 134, 351–357. [Google Scholar] [CrossRef]
- van de Scheur, F.T.; Staal, L.H. Effects of zinc addition to silica supported copper catalysts for the hydrogenolysis of esters. Appl. Catal. A General 1994, 108, 63–83. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, Z.; Zhang, W.; Sun, H.; Liu, S. Cu-Zn/Al2O3 Catalyst for the Hydrogenation of Esters to Alcohols. Chin. J. Catal. 2010, 31, 769–775. [Google Scholar] [CrossRef]
- Ding, G.; Zhu, Y.; Zheng, H.; Zhang, W.; Li, Y. Study on the reaction pathway in the vapor-phase hydrogenation of biomass-derived diethyl succinate over CuO/ZnO catalyst. Catal. Commun. 2010, 11, 1120–1124. [Google Scholar] [CrossRef]
- Pospelova, V.; Aubrecht, J.; Kikhtyanin, O.; Pacultová, K.; Kubička, D. CuZn Catalysts Superior to Adkins Catalysts for Dimethyl Adipate Hydrogenolysis. ChemCatChem 2019, 11, 2169–2178. [Google Scholar] [CrossRef]
- Pospelova, V.; Aubrecht, J.; Pacultová, K.; Lhotka, M.; Kikhtyanin, O.; Kubička, D. Does the structure of CuZn hydroxycarbonate precursors affect the intrinsic hydrogenolysis activity of CuZn catalysts? Catal. Sci. Technol. 2020, 10, 3303–3314. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Moribe, S.; Kanamori, Y.; Kakudate, M.; Takezawa, N. Preparation of a coprecipitated Cu/ZnO catalyst for the methanol synthesis from CO2—Effects of the calcination and reduction conditions on the catalytic performance. Appl. Catal. A General 2001, 207, 121–128. [Google Scholar] [CrossRef]
- Khassin, A.A.; Jobic, H.; Filonenko, G.A.; Dokuchits, E.V.; Khasin, A.V.; Minyukova, T.P.; Shtertser, N.V.; Plyasova, L.M.; Yurieva, T.M. Interaction of hydrogen with Cu–Zn mixed oxide model methanol synthesis catalyst. J. Mol. Catal. A Chem. 2013, 373, 151–160. [Google Scholar] [CrossRef]
- Le Valant, A.; Comminges, C.; Tisseraud, C.; Canaff, C.; Pinard, L.; Pouilloux, Y. The Cu–ZnO synergy in methanol synthesis from CO2, Part 1: Origin of active site explained by experimental studies and a sphere contact quantification model on Cu + ZnO mechanical mixtures. J. Catal. 2015, 324, 41–49. [Google Scholar] [CrossRef]
- Van den Berg, R.; Prieto, G.; Korpershoek, G.; van der Wal, L.I.; van Bunningen, A.J.; Laegsgaard-Jorgensen, S.; de Jongh, P.E.; de Jong, K.P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 2016, 7, 13057. [Google Scholar] [CrossRef]
- Durán-Martín, D.; Granados, M.L.; Fierro, J.L.G.; Pinel, C.; Mariscal, R. Deactivation of CuZn Catalysts Used in Glycerol Hydrogenolysis to Obtain 1,2-Propanediol. Top. Catal. 2017, 60, 1062–1071. [Google Scholar] [CrossRef]
- Küksal, A.; Klemm, E.; Emig, G. Reaction kinetics of the liquid-phase hydrogenation of succinic anhydride on CuZnO-catalysts with varying copper-to-zinc ratios in a three-phase slurry reactor. Appl. Catal. A General 2002, 228, 237–251. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Pospelova, V.; Aubrecht, J.; Lhotka, M.; Kubička, D. Effect of Calcination Atmosphere and Temperature on the Hydrogenolysis Activity and Selectivity of Copper-Zinc Catalysts. Catalysts 2018, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Aubrecht, J.; Pospelova, V.; Kikhtyanin, O.; Dubnová, L.; Kubička, D. Do metal-oxide promoters of Cu hydrogenolysis catalysts affect the Cu intrinsic activity? Appl. Catal. A General 2020, 608, 117889. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, X.; Chen, H.; Zheng, H.; Li, Y.-W.; Zhu, Y. Efficient Synthesis of Furfuryl Alcohol and 2-Methylfuran from Furfural over Mineral-Derived Cu/ZnO Catalysts. ChemCatChem 2017, 9, 3023–3030. [Google Scholar] [CrossRef]
- Millar, G.J.; Holm, I.H.; Uwins, P.J.R.; Drennan, J. Characterization of precursors to methanol synthesis catalysts Cu/ZnO system. J. Chem. Soc. Faraday Trans. 1998, 94, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Girgsdies, F.; Trunschke, A.; Schlögl, R. Minerals as Model Compounds for Cu/ZnO Catalyst Precursors: Structural and Thermal Properties and IR Spectra of Mineral and Synthetic (Zincian) Malachite, Rosasite and Aurichalcite and a Catalyst Precursor Mixture. Eur. J. Inorg. Chem. 2009, 2009, 1347–1357. [Google Scholar] [CrossRef]
- Fujita, S.-i.; Kanamori, Y.; Satriyo, A.M.; Takezawa, N. Methanol synthesis from CO2 over Cu/ZnO catalysts prepared from various coprecipitated precursors. Catal. Today 1998, 45, 241–244. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamamoto, K.; Ogura, N.; Katsumata, T.; Igarashi, A. Preparation of Cu/ZnO/Al2O3 catalyst for a micro methanol reformer. J. Power Sources 2005, 150, 20–26. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.; Song, H.; Chou, L. Characterization and performance of Cu/ZnO/Al2O3 catalysts prepared via decomposition of M(Cu, Zn)-ammonia complexes under sub-atmospheric pressure for methanol synthesis from H2 and CO2. J. Nat. Gas Chem. 2011, 20, 629–634. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Aubrecht, J.; Pospelova, V.; Kubička, D. On the origin of the transesterification reaction route during dimethyl adipate hydrogenolysis. Appl. Catal. A General 2020, 606, 117825. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The effect of ZnO in methanol synthesis catalysts on Cu dispersion and the specific activity. Catal. Lett. 1998, 56, 119–124. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Fan, G.; Li, F. Highly-Dispersed Copper-Based Catalysts from Cu–Zn–Al Layered Double Hydroxide Precursor for Gas-Phase Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Catal. Lett. 2012, 142, 1121–1127. [Google Scholar] [CrossRef]
- Rønning, M.; Huber, F.; Meland, H.; Venvik, H.; Chen, D.; Holmen, A. Relating catalyst structure and composition to the water–gas shift activity of Cu–Zn-based mixed-oxide catalysts. Catal. Today 2005, 100, 249–254. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sust. Energ. Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Ataya, F.; Dubé, M.A.; Ternan, M. Acid-Catalyzed Transesterification of Canola Oil to Biodiesel under Single- and Two-Phase Reaction Conditions. Energy Fuels 2007, 21, 2450–2459. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Aubrecht, J.; Pospelova, V.; Kikhtyanin, O.; Veselý, M.; Kubička, D. Critical Evaluation of Parameters Affecting Cu Nanoparticles Formation and Their Activity in Dimethyl Adipate Hydrogenolysis. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Pospelova, V.; Aubrecht, J.; Kikhtyanin, O.; Kubička, D. Towards efficient Cu/ZnO catalysts for ester hydrogenolysis: The role of synthesis method. Appl. Catal. A General 2021, 624, 118320. [Google Scholar] [CrossRef]
- Okamoto, Y.; Fukino, K.; Imanaka, T.; Teranishi, S. Surface characterization of copper(II) oxide-zinc oxide methanol-synthesis catalysts by x-ray photoelectron spectroscopy. 2. Reduced catalysts. J. Phys. Chem. 1983, 87, 3747–3754. [Google Scholar] [CrossRef]











| Sample | As-Prepared Samples | Calcined Samples | Spent Catalysts | |||||
|---|---|---|---|---|---|---|---|---|
| Targeted Cu/Zn Ratio | CuO by XRF, wt.% | ZnO by XRF, wt.% | Cu/Zn Ratio by XRF | BET Surface Area, m2·g−1 | Pore Volume, cm3·g−1 | BET Surface Area, m2·g−1 | Pore Volume, cm3·g−1 | |
| 0.5CuZn 1CuZn 3CuZn 6CuZn | 0.5 1 3 6 | 35.2 51.4 75.8 85.9 | 64.8 48.6 24.2 14.1 | 0.54 1.05 3.12 6.08 | 59 61 64 60 | 0.23 0.26 0.26 0.25 | 53 46 34 16 | 0.22 0.19 0.15 0.04 |
| Sample | Number of Acid Sites, mmol·g−1 | Number of Basic Sites, mmol·g−1 |
|---|---|---|
| 0.5CuZn-C 1CuZn-C 3CuZn-C 6CuZn-C | 0.135 0.136 0.168 0.151 | 0.176 0.187 0.237 0.190 |
| Sample | CuO Content (wt.%) by XRF | Consumed H2 (mmol·g−1) | CuO Content (wt.%) Calculated from Adsorbed H2 | SCu, m2·gcat−1 | SCu, m2·gCu−1 | Cu Dispersion, % |
|---|---|---|---|---|---|---|
| 0.5CuZn-C 1CuZn-C 3CuZn-C 6CuZn-C | 35.2 51.4 75.8 85.9 | 4.62 7.03 10.10 11.42 | 36.8 55.9 80.4 90.9 | 14.0 15.1 15.1 11.9 | 50 37 25 17 | 3.65 2.73 1.85 1.29 |
| Catalyst | MeHe Hydrogenolysis | HeHe Hydrogenolysis | TOFMeHe/ TOFHeHe | ||
|---|---|---|---|---|---|
| Initial Reaction Rate, rini (nMeHe·gcat−1·min−1) | TOF × 10−2 (s−1) | Initial Reaction Rate, rini (nHeHe·gcat−1·min−1) | TOF × 10−2 (s−1) | ||
| 0.5CuZn-R 1CuZn-R 3CuZn-R 6CuZn-R | 1.83 2.49 3.23 2.70 | 2.0 2.5 3.3 3.5 | 1.99 2.70 2.49 1.49 | 2.2 2.5 2.8 1.9 | 0.9 0.9 1.3 1.8 |
| Sample | ZnO Content in Catalysts, g | HeHe Yield (%) after 40 min | Formation Rate, mmolHeHe·gcat−1·min−1 | Corrected Formation Rate, mmolHeHe·gZnO−1·min−1 |
|---|---|---|---|---|
| ZnO 0.5CuZn-R 1CuZn-R 3CuZn-R 6CuZn-R | 0.75 0.97 0.72 0.36 0.20 | 4.45 1.40 1.46 1.89 3.61 | 0.82 0.13 0.13 0.17 0.33 | 0.82 0.20 0.28 0.73 2.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikhtyanin, O.; Aubrecht, J.; Pospelova, V.; Kubička, D. Understanding of the Key Factors Determining the Activity and Selectivity of CuZn Catalysts in Hydrogenolysis of Alkyl Esters to Alcohols. Catalysts 2021, 11, 1417. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111417
Kikhtyanin O, Aubrecht J, Pospelova V, Kubička D. Understanding of the Key Factors Determining the Activity and Selectivity of CuZn Catalysts in Hydrogenolysis of Alkyl Esters to Alcohols. Catalysts. 2021; 11(11):1417. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111417
Chicago/Turabian StyleKikhtyanin, Oleg, Jaroslav Aubrecht, Violetta Pospelova, and David Kubička. 2021. "Understanding of the Key Factors Determining the Activity and Selectivity of CuZn Catalysts in Hydrogenolysis of Alkyl Esters to Alcohols" Catalysts 11, no. 11: 1417. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111417