1. Introduction
In recent years, due to the shortage of non-renewable energy resources and increasing environmental pressure from greenhouse gases released by burning of fossil fuels, there has been an increase in the number of studies searching for alternative production of cleaner fuels compared to petroleum-based fuels. Second-generation biofuels are produced from lignocellulosic biomass from agricultural and forestry residues, which are considered bountiful sources that neither compete with food requirements nor impact the food and feed markets [
1]. Sugarcane bagasse is one of the most abundant and promising biomass sources in the world, obtained from the processing of sugarcane with an estimated global yield of 510.3 million tons per year [
2].
Sugarcane bagasse is predominantly composed of cellulose, hemicellulose, and lignin, which are strongly associated to form the plant cell wall, resulting in a stable and recalcitrant structure [
3]. Cellulose and hemicellulose are polysaccharides that cannot be directly converted into ethanol but can be hydrolyzed to obtain monosaccharides such as glucose and xylose that, in turn, can be fermented to produce ethanol [
4,
5]. Enzymatic hydrolysis is applied to convert cellulose to glucose (substrate used in the classic production of first-generation ethanol) [
6]. Once pre-treated, lignocellulosic biomass presents less hemicellulose and lignin, which makes cellulose more accessible to enzymes, increasing the hydrolysis efficiency [
7,
8].
Recently, many studies have reported the use of different combinations of enzymes and enzymatic cocktails composed of three or more enzymes [
9,
10,
11,
12,
13]. Endoglucanase (EC 3.2.1.4) in synergy with
β-glucosidase (EC 3.2.1.21) are commonly used in enzymatic hydrolysis of pretreated lignocellulosic biomass, due to their combined action: Endoglucanases catalyze the hydrolysis of cellulose to cellobiose, which then serves as substrate for glucose production by the action of
β-glucosidases [
12,
14,
15].
The pretreated sugarcane bagasse saccharification by enzymatic hydrolysis is an expensive process mostly due to the cost of the applied enzymes, whereby the reuse of these biocatalysts is an interesting means to reduce this cost. For years, several enzymatic immobilization techniques have been used with well-established protocols in order to enable not only the reuse of these biocatalysts, but also to improve their activity and stability, to prevent enzymes from aggregating and undergoing autolysis, and to increase flexibility of the reactor design [
16,
17].
It is possible to immobilize two or more enzymes on the same support forming a multi-enzymatic system to be applied in cascade reactions. A disadvantage of this immobilization method is that the immobilized derivative can only be applied to the same reaction and, if the enzymes have different stability, the derivative loses efficiency with the loss of activity of at least one of the enzymes [
18]. Thus, the supports used to immobilize each enzyme must be chosen considering both the material (porous or non-porous) and the recovery method at the end of each reaction cycle. Using endocellulases and
β-glucosidases as catalysts in hydrolysis, the first enzyme must not be immobilized in porous supports because it will not be able to access the insoluble substrate (cellulose), unlike the second enzyme that hydrolyzes a soluble substrate (cellobiose) [
19].
Among the several existing enzyme immobilization protocols, the method performed by multipoint covalent link on activated supports has been reported as the most promising to improve the stability of the catalyst since, once immobilized, the links between the active groups of the support and the enzyme are maintained providing resistance to any conformational change [
17]. In this context, glyoxyl agarose is considered one of the most suitable supports providing higher stability to the immobilized enzyme, allowing a recovery of activity between 60 and 100% [
20].
Immobilization techniques using magnetic nanoparticles as non-porous support have attracted much attention due to properties such as cost and ease in the synthesis of the support [
21], better enzyme performance by biocompatibility, high surface area availability, and the simplicity of recovery using an external magnetic field [
22], which can increase the recycling of both the support and the immobilized derivative [
23].
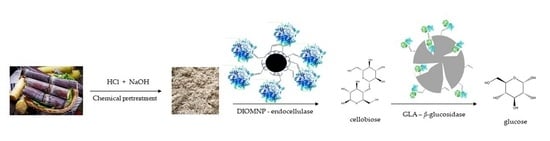
Thus, this study aims to immobilize an endocellulase and a β-glucosidase (both commercial) on different supports, respectively. Dextran coated Fe2O3 with aldehyde groups were used as a non-porous support to immobilize endocellulase and β-glucosidase was immobilized on glyoxyl agarose. The derivatives were characterized concerning thermal stability and applied in saccharification of pretreated sugarcane bagasse to obtain a glucose-rich hydrolyzate. For the first time, endocellulase was immobilized on the non-porous support dextran coated Fe2O3 with aldehyde groups, to be applied in combination with a β-glucosidase immobilized on glyoxyl agarose for sugarcane bagasse saccharification by cascade reaction.
2. Results and Discussion
2.1. Immobilization of Endocellulase and β-Glucosidase on Different Supports
Initially, a load of 35 U/g of support (E-CELBA) and 30.67 U/g of support (E-BGOSPC) were used for immobilization. The immobilization was carried out in glyoxyl agarose at pH 10.0 and room temperature. Immobilization times of 1, 2, and 4 h of incubation were evaluated and after each time, Schiff bases were reduced to form covalent bonds and to ensure that the remaining aldehyde groups of the support were converted to hydroxyls [
24].
Table 1 shows the results of immobilization yield and specific activity before and after the reduction with NaBH
4 for 1, 2, and 4 h of immobilization of each enzyme at room temperature. Almost 90% of E-CELBA was immobilized on dextran coated iron oxide magnetic nanoparticles (DIOMP) in the first hour, but after reduction, the derivative presents only 37.7% of immobilized activity representing 11.72 ± 0.21 U/g of support. With 2 h of incubation, 97.76 ± 2.21% of the enzyme was immobilized with 51.4% activity loss after reduction. However, after 4 h of incubation, 96.56 ± 3.40% of E-CELBA was immobilized retaining 65.21% of its activity (22.04 ± 0.42 U/g of support) after the reduction. The difference in enzymes activity after reduction, at different immobilization times, could be explained by the change in type of enzyme–support interaction over time. At the beginning of immobilization on supports activated with aldehyde groups, although the first bonds between the aldehyde group and primary amino groups of the enzyme are made quickly, they are weak and can be broken making them reversible; over time, additional bonds are made, increasing the quantity of bonds between the enzyme and support, leaving its structure more rigid, resulting in stability gains to the immobilized derivative [
20,
25].
Results of E-BGOSPC immobilization on GLA were not as expressive as for endocellulase, obtaining an immobilization of only 13.71 ± 1.12% and 3.16 ± 0.11% after the reduction (
Table 1). On the other hand, E-BGOSPC presents lower stability in alkaline pH (manufacturer’s information), which may have interfered during the immobilization process. An enzyme immobilization hardly occurs at slightly alkaline pH values due to the instability generated by the Schiff bases formation between the enzyme and support. However, in the presence of thiolated compounds (e.g.,
N-acetylcysteine) these bonds are stabilized without irreversible reduction [
26]. Thus, in order to decrease the immobilization pH in GLA (<9.0) without sacrificing support reactivity,
N-acetylcysteine was used as a pH-reducing agent in the immobilization of E-BGOSPC and the results are shown in
Table 2.
In the presence of 50 mM N-acetylcysteine at pH 8.5 and room temperature, in 1 h of immobilization, more than 50% of the enzyme was immobilized. After 4 h 98.17 ± 1.12% of E-BGOSPC was immobilized on GLA, maintaining 66.96 ± 0.76% of activity (20.54 ± 0.28 U/g of support) after reduction. These results prove that N-acetylcysteine can be used as a pH-reducing agent to improve enzyme immobilization on glyoxyl supports under mild pHs.
Although immobilized DIOMNP and GLA derivatives showed loss of specific activity after reduction with NaBH
4, the results obtained in this work are similar and promising when compared to the results of other works published in the literature. Paz-Cedeno et al. [
27] studied the immobilization of endocellulase and
β-glucosidase on graphite oxide-magnetite reaching 63.4% and 97.2% of immobilization respectively. Endocellulase from
Scytalidium thermophilum and
β-glucosidase from
Humicola insolens were immobilized on magnetic nanoparticles with chitosan activated with NiCl
2, reaching a 20% immobilization yield for both enzymes [
28]. Nishida et al. [
29] studied the immobilization of
Aspergillus awamori β-glucosidase on crosslinked commercial gelatin glutaraldehyde, at 4 °C, reaching 99.8% immobilization and presenting 62.5% of the initial enzymatic activity after 2 h of incubation.
2.2. Thermal Stability of Soluble Enzymes and Immobilized Derivatives
Thermal stability tests of both soluble enzymes E-CELBA and E-BGOSPC, and their derivatives immobilized on DIOMNP and GLA, respectively, were carried out in 25 mM sodium phosphate buffer pH 5.0 and 7.0, incubating samples at 60, 70, and 80 °C.
Figure 1 shows the results obtained in the experiments.
For all temperatures studied, soluble E-CELBA showed lower stability at pH 5.0, reaching total inactivation in 6 h at 60 °C. Whereas, soluble E-BGOSPC, after 72 h incubated at this temperature, a residual activity of 19.43 ± 0.86% was obtained. At temperatures of 70 and 80 °C, both soluble enzymes were completely inactivated in the first 3 h of incubation. At pH 7.0 and 60 °C, soluble E-BGOSPC was less stable than soluble E-CELBA incubated at 60 °C. At 6 h incubation, β-glucanase showed a residual activity of 31.11 ± 1.08% with an inactivation of more than 95% in 24 h, while endocellulase showed 87.05 ± 1.19% of residual activity after 6 h and retained 31.97 ± 0.99% of residual activity in 72 h of incubation. Both enzymes showed similar thermostability at 70 °C (approximately 50% inactivation after 4 h) and 80 °C (approximately 50% inactivation after 2 h).
At 60 °C (
Figure 1a), both derivatives showed residual activities superior to 80% at 72 h of incubation at both pH conditions. After 144 h incubation, DIOMNP derivative remained approximately the 62% residual activity at pH 5.0 and 7.0. At pH 5.0, GLA derivative was more stable, inactivating approximately 19% of biocatalyst after 144 h of incubation.
After 72 h of incubation at 70 °C (
Figure 1b) and pH 5.0, both derivatives showed similar stability profiles. DIOMNP derivative lost half of its initial activity in 48 h incubation at both pHs. The same behavior was observed for GLA derivative incubated at pH 5.0. On the other hand, when incubated at pH 7.0, GLA derivative lost around 61% of its activity in 24 h of incubation.
At 80 °C (
Figure 1c), DIOMNP derivative showed 50.43 ± 0.78% of residual activity, while GLA derivative showed 36.48 ± 0.63% residual activity in 24 h of incubation at pH 5.0. At pH 7.0, DIOMNP was more stable compared to GLA derivative. After 6 h of incubation, DIOMNP derivative maintained 77.37 ± 1.21% of initial activity while the GLA derivative maintained only 40.10 ± 1.09%. Although after 72 h of incubation at pH 5.0 and 7.0, both derivatives were more than 90% inactivated, DIOMNP derivative showed residual activity 10-times higher than the GLA derivative at pH 7.0.
The results obtained in this study with E-CELBA and E-BGOSPC enzymes show that the enzymatic immobilization significantly improves the thermal stability of obtained biocatalysts.
2.3. Saccharification of Sugarcane Bagasse by Enzymatic Hydrolysis
After immobilization and stabilization of endocellulase in DIOMNP and
β-glycosidase in GLA, the derivatives were applied to saccharification of pretreated sugarcane bagasse to obtain a glucose-rich hydrolyzate, under the conditions described in
Section 3.7. Soluble enzymes were applied under the same conditions for control.
After the reaction medium reached the desired temperature, hydrolysis was started by the addition of 0.68 g of DIOMNP and 0.72 g of GLA derivatives for each g of pretreated bagasse. For the control condition, 10.71 μL of soluble E-CELBA and 32.61 μL of E-BGOSPC were added for each gram of pretreated bagasse.
Table 3 shows the concentrations of sugars present in the hydrolyzate, as well as the cellulose conversions obtained in process catalyzed by derivatives and soluble biocatalysts (calculated according to Equation 1) after 48 h of reaction.
The control hydrolysis was carried out with soluble E-CELBA and E-BGOSPC, and the hydrolysis with the derivatives DIOMNP and GLA, both with enzymatic activity equivalent to 15 U/g of bagasse, presented similar results when comparing the concentration of reducing sugars (cellobiose and glucose) and the cellulose conversion.
For the control reaction, 41.02 ± 1.32% of the cellulose present in the reaction was converted totalizing 14.81 ± 0.54 g/L of reducing sugars, where 94.40% was glucose. For the hydrolysis with immobilized derivatives, 13.34 ± 0.43 g/L of glucose were produced (94.54% of the whole amount of reducing sugars), totalizing 14.11 ± 0.47 g/L of reducing sugars produced by converting, approximately, 39.07 ± 1.18% of the cellulose present in the reaction. This result was approximately 5% lower than the amount of reducing sugars achieved in the control hydrolysis catalyzed by soluble enzymes. In both tests, cellobiose concentration was less than 1.0 g/L after 48 h, which indicates that the load of E-BGOSPC and its GLA derivative used was adequate to convert cellobiose (released from cellulose by the action of E-CELBA and its derivative DIOMNP) in glucose.
Studies on sugarcane bagasse saccharification by enzymatic hydrolysis have been reported in literature and show promising results. Zhang et al. [
30] carried out the hydrolysis of sugarcane bagasse pretreated using NaOH and ethanol, in an autoclave, and they obtained 88.2% cellulose conversion into reducing sugars (61.9% glucose) after 24 h of reaction. These results were achieved with 40 U of Novozymes soluble active cellulose applied in a reaction medium containing 2.0% pretreated sugarcane bagasse and supplemented with Tween 80. Mukasuru et al. [
11] studied the hydrolysis of sugarcane bagasse pretreated with glycerol in a fed-batch system using a concentration of 3.0 U/g Cellic CTec2 cellulose (Novozymes) and accessory enzymes in a medium containing additives such as Tween 80, tea saponin and BSA, achieving 63.0% of cellulose conversion with 180 g/L of reducing sugars, of which 40% are glucose. In another study, sugarcane bagasse (pretreated with glycerol) was saccharified using an enzyme cocktail containing cellulases, hemicellulases, and
β-glucosidase from fungus
Lichtheimia ramosa and, after 24 h of reaction, 10.66% of the cellulose was converted into reducing sugars, of which only 1.45 g/L was glucose [
31].
The glucose yield and cellulose conversion obtained in the present study are promising and even more effective when compared with other similar works. In order to improve the results obtained, an optimization of the concentrations of E-CELBA and E-BGOSPC immobilized in DIOMNP and GLA, respectively, will be carried out in future work, as well as the solid–liquid ratio to obtain better yields from the conversion of cellulose to glucose.
2.4. Derivatives Reuse
Industrial-scale processes using enzymes are expensive and often economically unviable due to the high cost of these biocatalysts and the impossibility of being reused as they present low stabilities and cannot be recovered. The main advantage of using immobilized enzymes in these processes is the possibility of recovery and reuse the same portion of each biocatalyst in new processes, both in batch and continuous operation. Therefore, the reuse of the derivatives DIOMNP (endocellulase) and GLA (
β-glycosidase) was studied submitting the derivatives to successive CMC hydrolysis cycles (for 48 h each cycle) at 50 °C (
Figure 2).
The DIOMNP derivative maintained 80% of its initial activity in the first five cycles (
Figure 2a), exhibiting a residual activity of 94.7 ± 0.71 and 90.3 ± 0.93% in cycles 2 and 3, respectively. From the cycle 6, DIOMNP showed a marked and constant activity decrease after each subsequent reaction cycle, retaining more than 50% of the residual activity until cycle 8, and inactivating almost 75% after 10 reaction cycles. The GLA derivative presented a similar profile to the DIOMNP derivative in the first five cycles maintaining more than 80% of its initial activity, with 98.2 ± 2.27% and 90.6 ± 1.65% of residual activity in cycles 2 and 3, respectively. However, in cycles 6 and 7, the GLA maintained more than 75% of its initial activity, retaining 58.2 ± 1.55% of the residual activity until cycle 9, and inactivating almost 60% after 10 reaction cycles. All residual activity values presented by the GLA derivative were higher than those presented by the DIOMNP derivative after each reaction cycle.
Activity losses may be related to the release of enzymes from the supports due to the continuous recovery steps for each of the derivatives and by enzyme inactivation due to the prolonged time of exposure to process parameters such as temperature and pH.
Studies about immobilization of endocellulase and
β-glucosidase on different supports and applying different strategies show the possibility to reuse of immobilized derivatives, conserving between 40 and 90% of the initial enzymatic activity. Among the several studies reported, recently Paz-Cedeno et al. [
27] used graphite oxide-magnetite to immobilize endocellulase and
β-glucosidase resulting in stable derivatives capable of maintaining 70% and 88% of their initial enzymatic activities, respectively, after 10 cycles of reuse. Abraham et al. [
32] immobilized cellulases on magnetite nanoparticles using glutaraldehyde resulting in a stable derivative capable of maintaining 70% of its initial enzymatic activity after 10 consecutive hydrolysis cycles. On the other hand,
β-glucosidase from
Humicola insolens immobilized on magnetic nanoparticles derivatized with chitosan activated with NiCl
2, maintained only 50% of its initial activity after the third cycle of reuse. Endocellulase from
Scytalidium thermophilum immobilized on the same support, maintained approximately 45% of its activity after the second cycle and inactivated 90% after the fifth cycle [
28]. The results presented show that the support and the protocol used in the immobilization can change the way the enzyme is linked on support, resulting in more stable biocatalysts in relation to the reaction parameters, and in order to avoid the release of the enzyme from the support during the recovery steps, thereby increasing the amount of reuse of the derivative.
3. Materials and Methods
3.1. Pretreated Sugarcane Bagasse
The sugarcane bagasse was kindly provided by sugarcane refinery Jalles Machado S.A, Goiás, Brazil. The bagasse was pretreated with diluted hydrochloric acid (6.0%
v/
v) using 50 mL of acidic solution for each 3.3 g of bagasse kept under mild agitation at 96.8 °C for 375 min. After that, the solid fraction was filtered, washed with distilled water, and delignified with a NaOH solution (2%
v/
v water) in autoclave at 121 °C for 30 min. Then the resulting solid fraction was filtered, washed with distilled water, and dried. After delignification, the carbohydrate content in the pre-treated sugarcane bagasse was determined according to the NREL procedure [
33] presenting a composition as follows: 65.19 ± 0.68% cellulose; 2.2 ± 0.07% lignin; 21.54 ± 0.16% extractives and ashes, 11.03 ± 0.71% moisture and absence of hemicellulose.
3.2. Enzymes
Endocellulase (endo-1,4-β-d-glucanase) from Bacillus amyloliquefaciens (E-CELBA) and β- glucosidase from Phanerochaete chrysosporium (E-BGOSPC) were purchased from Megazyme Develops, Manufactures & Supplies Analytical Solutions. According to information provided by the manufacturer, endocellulose and β-glucosidase activities were 1400 U/mL and 460 U/mL, respectively.
3.3. Enzyme Assays
3.3.1. Endocellulase Activity
E-CELBA activity was determined using carboxymethyl-cellulose (CMC) as the substrate and according to a modified reducing sugar method of analysis [
34]. Briefly, 25 μL of 1:10 enzymatic solution (
v/
v soluble enzyme;
w/
v immobilized derivative) were added to 50 μL of 2% CMC solution prepared in 100 mM sodium phosphate buffer pH 6.0. After 15 min of reaction at 50 °C, 150 μL of 3,5-dinitrosalicylic acid (DNS) solution [
35] were added and the mixture was boiled for 10 min, cooled, and measured using a spectrophotometer at 540 nm. A unit (U) of enzymatic activity was defined as the amount of enzyme required to produce 1.0 μmole of reducing sugars per minute, under the assay conditions. A standard calibration curve was defined from serial glucose dilutions.
3.3.2. β-Glucosidase Activity
E-GOSPC activity was analyzed by a method proposed by Hang and Woodams [
36] modified using
p-nitrophenyl-
β-
d-glucopyranoside (
pNPG) as the substrate. In 100 μL of the 1:10 enzyme solution (
v/
v soluble enzyme;
w/
v immobilized derivative), 90 μL of 250 mM sodium citrate buffer pH 4.5 and 10 μL of
pNPG (4.0 mg/mL) were added. After incubation at 37 °C for 10 min, 1.0 mL of 500 mM sodium carbonate buffer pH 10 was added to stop the reaction. Then the activity was measured by spectrophotometer at 405 nm. A unit (U) of enzymatic activity was defined as the amount of enzyme required for the hydrolysis of 1.0 μmole of
pNP per minute, under the assay conditions, considering ε = 18,700.
3.4. Support Preparation
3.4.1. Glyoxyl-Agarose
Glyoxyl-agarose (GLA) support was prepared as described by Guisán [
37]. Agarose (10 BCL) activation was carried out by adding 105 g of washed and filtered agarose in 30 mL of distilled water and 50 mL of 1.7 M sodium hydroxide (NaOH) solution containing 1.425 g of sodium borohydride (NaBH
4). The solution was kept in an ice bath under magnetic stirring. Then, 36 mL of glycidol was slowly added and kept under mechanical stirring at room temperature overnight. Sodium periodate (NaIO
4) was used as oxidizing agent to reduce the hydroxyl groups of glyceryl-agarose (agarose activated with glycidol) into aldehyde groups. To 70 g of glyceryl-agarose, 25 mL of NaIO
4 were added and the suspension was kept under mild agitation at room temperature for 2 h. Next, the obtained GLA was thoroughly washed with distilled water and filtered.
3.4.2. Dextran Coated Iron Oxide Magnetic nanoparticles Activated with Aldehyde Groups
Magnetic nanoparticles were synthesized using a 4-step protocol with Fe
2O
3 as core (12 nm), coating the surface with dextran from
Leuconostoc spp. (~40 kDa) and activating it to obtain aldehyde functional groups. The magnetic nanoparticles synthesis was carried out following the Massart co-precipitation protocol [
38]. An optimized oxidizing treatment using HNO
3 was applied in order to improve the magnetic nanoparticles properties and colloidal stability resulting in nanoparticles with a size distribution below 0.2 μm of the polydispersity degree [
39].
Dextran coating on the nanoparticles surface was performed as described by Bautista et al. [
40]. A solution prepared by dropwise addition of 10 mL of nanoparticles dispersion with 228 mg Fe
2O
3/mL in 1.6 mL of 0.8 M NaOH was mixed with a solution containing 200 mg dextran in 2.5 mL of 0.5 M NaOH. The mixture was mechanically stirred for 24 h.
The crosslinking step to increase dextran coated nanoparticles stability was carried out using glycidol. For that purpose, 9 mL of nanoparticle colloid were added to 9 mL of a 5 M NaOH solution and 1.5 mL glycidol (96% solution). The reaction occurred under continuous agitation for 24 h at room temperature. Then, to remove glycidol excess, the nanoparticles were magnetically separated and dialyzed against deionized water for 24 h.
Finally, to activate the dextran coated magnetic nanoparticles with aldehyde groups, 0.25 mL of 25 mM NaIO4 was used to oxidize 1 mL of nanoparticles suspension. The reaction was carried out in the absence of light and oxygen, under mechanical agitation at 150 rpm for 4 h. Then 0.2 mL of 2 M ethylene glycol was added and left under stirring for 30 min. To remove periodate excess, the nanoparticles were magnetically separated and dialyzed against deionized water for 24 h.
Dextran coated magnetic nanoparticles activated with aldehyde groups (DIOMNP) were stored in distilled water in the concentration of 10 mg DIOMNP/mL.
3.5. Enzymes Immobilization
For DIOMNP, before initiating the immobilization, the stock suspension containing the support (10 mg/mL) was sonicated for 15 s to undo possible aggregation that may have occurred during storage. Then, 10 mL of E-CELBA solution diluted 1:400 (v/v) in 25 mM sodium bicarbonate buffer pH 10 were added to 0.5 mL of support suspension (5.0 mg of DIOMNP). For GLA, 10 mL of E-BGOSPC solution diluted 1:150 (v/v) in 25 mM sodium bicarbonate pH 10 were added to 1.0 g of support. Suspensions were left under mild stirring at room temperature. Supernatant and suspension samples were periodically taken and their enzymatic activities were measured. Then, the preparations were washed with distilled water. At pH 8.5, E-BGOSPC was diluted (1:150 v/v) in a solution of 50 mM N-acetylcysteine prepared in 25 mM sodium bicarbonate pH 8.5. Then, 10 mL of enzyme solution were added to 1.0 g of GLA.
After the immobilization of each enzyme on different supports, NaBH
4 was used to achieve the final reduction by transforming the remaining aldehydes into inert hydroxyl groups and weak Schiff bases into highly stable secondary amino bonds [
24]. Thus, 1.0 mg of NaBH
4 was added to each mL of the immobilization suspension and kept under stirring for 30 min at room temperature. The biocatalysts were vacuum filtered and thoroughly washed with distilled water.
3.6. Thermal Stability
According to information provided by the manufacturer, both enzymes are stable at temperatures up to 60 °C. Thus, thermal stability evaluation was carried out by incubating the soluble enzymes and their counterpart derivatives (1:400 (v/v) to soluble endocellulase, 1:150 (v/v) to soluble β-glucosidase, and 1:10 (w/v) to derivatives diluted in 25 mM sodium phosphate buffer) at 60, 70, and 80 °C, in pH pH 5.0 and 7.0 for 72 h and sampling them periodically. Enzymatic activities were determined using the standard assay as previously described. The residual activities were calculated by comparing the final enzymatic activity (after the incubation) to the initial activity (before incubation corresponding 100%).
3.7. Saccharification of Pretreated Sugarcane Bagasse
Saccharification of pretreated sugarcane bagasse was performed with soluble endocellulase and β-glucosidase (control) and its immobilized derivatives. The reaction was conducted with pretreated bagasse and 100 mM sodium acetate buffer pH 5.5 in a 1:20 liquid–solid ratio. In order to equalize the reaction temperature, the reaction medium was kept under magnetic stirring at 50 °C for 30 min before enzyme addition. Then, hydrolysis was initiated with the addition of soluble enzymes or derivatives, both corresponding to 15 U/g of bagasse. The reaction occurred under mild magnetic stirring at 50 °C for 48 h. To stop the hydrolysis after the operating time, the reaction mixture with soluble enzymes was kept at 100 °C for 10 min. In the case of derivatives, hydrolysis was stopped through recovering the catalysts from the reaction mixture either magnetically (for DIOMNP) or by filtration (for GLA). The liquid fraction was analyzed by HPLC coupled with refractive index detector RID 10-A using a reverse-phase column (Aminex® HPX87H). Products were eluted using a solution of 5 mM H2SO4 as mobile phase at a flow rate of 0.6 mL/min and 45 °C. The sugar concentrations were determined using calibration curves.
After quantifying the sugars present in enzymatically saccharified bagasse hydrolyzate, the cellulose conversion (
Cc) was calculated as described in Equation (1):
where
Cglucose and
Ccellobiose are, respectively, glucose and cellobiose concentration (g/L) in the enzymatic hydrolyzate; 0.9 and 0.95 are stoichiometric factors considering the molecular mass between glucose and cellobiose, respectively;
Cbagasse is the concentration (g/L) of pretreated sugarcane bagasse used in hydrolysis; and
Ccellulose.bagasse is the cellulose content (65.19 ± 0.68%) in pretreated sugarcane bagasse.
3.8. Derivatives Reuse
Derivatives reuse was performed in batch assays by incubating 0.1 g of each derivative, with 1.0 mL of 2% CMC prepared with 50 mM sodium acetate buffer pH 5.5 at 50 °C. After complete hydrolysis, DIOMNP derivative was magnetically recovered and the remaining suspension was centrifuged for 1 min at 3500 rpm, to recover the GLA. Then, the hydrolytic activity of derivatives was measured, separately, according to the procedure described in
Section 2.3. After each cycle, the derivatives were recovered, washed with 25 mM sodium phosphate buffer pH 7.0, filtrated, and added to a new hydrolysis cycle with a new substrate solution. The activity of each cycle was expressed in relation to the activity after the first cycle (corresponding to 100%).
4. Conclusions
In summary, endocellulase immobilized on magnetic nanoparticles and β-glucosidase immobilized on glyoxyl agarose is a good strategy to reduce costs of the sugarcane bagasse saccharification process in order to obtain a glucose-rich hydrolyzate for ethanol production by submerged fermentation. According to the results presented in this study, the immobilized enzymes E-CELBA and E-BGOSPC had a significant thermal stability improvement. During sugarcane bagasse enzymatic hydrolysis, the results obtained using soluble enzymes and immobilized derivatives were similar, approximately 0.27 g glucose/g bagasse. However, the immobilized derivatives could be used for several consecutive hydrolysis cycles, without significant loss of activity. Specifically, DIOMNP endocellulase derivative maintained more than 60% of its enzymatic activity in seven reuse cycles, and GLA β-glucosidase derivative maintained more than 58% of its enzymatic activity after 8 reuse cycles.