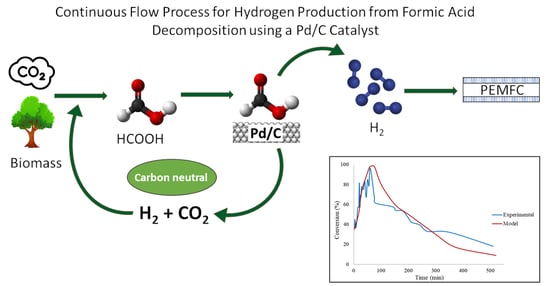
Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Results
2.1.1. Model Validation
2.1.2. Effects of Temperature and Liquid Flow Rate
2.1.3. Internal and External Mass Transfer Resistances
2.1.4. Comparison of Packed Bed Microreactor and Batch Stirred Reactor
2.2. Experimental Results
2.2.1. Catalyst Reusability
2.2.2. Catalyst Characterisation
3. Experimental Methodology
3.1. Materials and Chemicals
3.2. Catalyst Characterisation
3.3. Experimental Set-Up
4. Modelling of Packed Bed Microreactor
4.1. Reaction Kinetics
4.2. Numerical Procedure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrog. Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.H. Understanding the effect of Pd size on formic acid dehydrogenation via size-controlled Pd/C catalysts prepared by NaBH4 treatment. Appl. Catal. B Environ. 2019, 244, 684–693. [Google Scholar] [CrossRef]
- Barnard, J.H.; Wang, C.; Berry, N.G.; Xiao, J. Long-range metal-ligand bifunctional catalysis: Cyclometallated iridium catalysts for the mild and rapid dehydrogenation of formic acid. Chem. Sci. 2013, 4, 1234–1244. [Google Scholar] [CrossRef]
- Gao, Y.; Kuncheria, J.K.; Jenkins, H.A.; Puddephatt, R.J.; Yap, G.P. The interconversion of formic acid and hydrogen/carbon dioxide using a binuclear ruthenium complex catalyst. J. Chem. Soc. Dalton Trans. 2000, 18, 3212–3217. [Google Scholar] [CrossRef]
- Zell, T.; Butschke, B.; Ben-David, Y.; Milstein, D. Efficient Hydrogen Liberation from Formic Acid Catalyzed by a Well-Defined Iron Pincer Complex under Mild Conditions. Chem. Eur. J. 2013, 19, 8068–8072. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Liu, J.; Murphy, C.J.; Liriano, M.L.; Wasio, N.A.; Lucci, F.R.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective formic acid dehydrogenation on Pt-Cu single-atom alloys. ACS Catal. 2017, 7, 413–420. [Google Scholar] [CrossRef]
- Mielby, J.; Kunov-Kruse, A.J.; Kegnæs, S. Decomposition of formic acid over silica encapsulated and amine functionalised gold nanoparticles. J. Catal. 2017, 345, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, W.; Zhao, H.; Zheng, X.; Wu, L.; Pan, H.; Zhu, J.; Chen, Y.; Lu, J. Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid. J. Catal. 2017, 352, 371–381. [Google Scholar] [CrossRef]
- Sanchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen generation from additive-free formic acid decomposition under mild conditions by Pd/C: Experimental and DFT studies. Top. Catal. 2018, 61, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhu, Q.-L.; Tsumori, N.; Xu, Q. Immobilizing highly catalytically active noble metal nanoparticles on reduced graphene oxide: A non-noble metal sacrificial approach. J. Am. Chem. Soc. 2015, 137, 106–109. [Google Scholar] [CrossRef]
- Tang, C.; Surkus, A.E.; Chen, F.; Pohl, M.M.; Agostini, G.; Schneider, M.; Junge, H.; Beller, M. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid. Angew. Chem. 2017, 129, 16843–16847. [Google Scholar] [CrossRef]
- Du, Y.; Shen, Y.-B.; Zhan, Y.-L.; Ning, F.-D.; Yan, L.-M.; Zhou, X.-C. Highly active iridium catalyst for hydrogen production from formic acid. Chin. Chem. Lett. 2017, 28, 1746–1750. [Google Scholar] [CrossRef]
- Zacharska, M.; Bulusheva, L.G.; Lisitsyn, A.S.; Beloshapkin, S.; Guo, Y.; Chuvilin, A.L.; Shlyakhova, E.V.; Podyacheva, O.Y.; Leahy, J.J.; Okotrub, A.V.; et al. Factors influencing the performance of Pd/C catalysts in the green production of hydrogen from formic acid. ChemSusChem 2017, 10, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-S.; Song, J.; Song, M.; Goo, B.S.; Lee, Y.W.; Kim, Y.; Yang, H.; Han, S.W. Core-shell engineering of Pd–Ag bimetallic catalysts for efficient hydrogen production from formic acid decomposition. ACS Catal. 2018, 9, 819–826. [Google Scholar] [CrossRef]
- Akbayrak, S. Decomposition of formic acid using tungsten(VI) oxide supported AgPd nanoparticles. J. Colloid Interface Sci. 2019, 538, 682–688. [Google Scholar] [CrossRef]
- Zhao, Z.; Heck, K.N.; Limpornpipat, P.; Qian, H.; Miller, J.T.; Wong, M.S. Hydrogen-generating behavior of Pd-decorated gold nanoparticles via formic acid decomposition. Catal. Today 2019, 330, 24–31. [Google Scholar] [CrossRef]
- Duan, S.; Zhang, S.; Chang, S.; Meng, S.; Fan, Y.; Zheng, X.; Chen, S. Efficient photocatalytic hydrogen production from formic acid on inexpensive and stable phosphide/Zn3In2S6 composite photocatalysts under mild conditions. Int. J. Hydrog. Energy 2019, 44, 21803–21820. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Rostamnia, S.; Hassankhani, A.; Liu, X.; Eftekhari, A.; Hasanzadeh, A.; Zhang, K.; Karimi-Maleh, H.; Khaksar, S.; Varma, R.S.; et al. Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-MIL-101: Synergic boost to hydrogen production from formic acid. J. Colloid Interface Sci. 2020, 567, 126–135. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Nie, W.; Yao, Q.; Zhang, Z.; Lu, Z.-H. Anchoring IrPdAu Nanoparticles on NH2-SBA-15 for Fast Hydrogen Production from Formic Acid at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 8082–8090. [Google Scholar] [CrossRef]
- Boddien, A.; Loges, B.; Junge, H.; Gärtner, F.; Noyes, J.R.; Beller, M. Continuous hydrogen generation from formic acid: Highly active and stable ruthenium catalysts. Adv. Synth. Catal. 2009, 351, 2517–2520. [Google Scholar] [CrossRef]
- Majewski, A.; Morris, D.J.; Kendall, K.; Wills, M. A Continuous-Flow Method for the Generation of Hydrogen from Formic Acid. ChemSusChem 2010, 3, 431–434. [Google Scholar] [CrossRef]
- Sponholz, P.; Mellmann, D.; Junge, H.; Beller, M. Towards a practical setup for hydrogen production from formic acid. ChemSusChem 2013, 6, 1172–1176. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Pronk, F.; Bril, T.; Wenn, D.; Shaw, J.; Gavriilidis, A. CO2 absorption in a high efficiency silicon nitride mesh contactor. Chem. Eng. J. 2012, 207, 766–771. [Google Scholar] [CrossRef]
- Hafeez, S.; Manos, G.; Al-Salem, S.; Aristodemou, E.; Constantinou, A. Liquid fuel synthesis in microreactors. React. Chem. Eng. 2018, 3, 414–432. [Google Scholar] [CrossRef]
- Sun, X.; Constantinou, A.; Gavriilidis, A. Stripping of acetone from isopropanol solution with membrane and mesh gas-liquid contactors. Chem. Eng. Process. Process Intersif. 2011, 50, 991–997. [Google Scholar] [CrossRef]
- Wu, G.; Cao, E.; Ellis, P.; Constantinou, A.; Kuhn, S.; Gavriilidis, A. Continuous flow aerobic oxidation of benzyl alcohol on Ru/Al2O3 catalyst in a flat membrane microchannel reactor: An experimental and modelling study. Chem. Eng. Sci. 2019, 201, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Tang, Y.; Pan, M.; Wei, X.; Chen, H.; Xiang, J. A performance study of methanol steam reforming microreactor with porous copper fiber sintered felt as catalyst support for fuel cells. Int. J. Hydrog. Energy 2009, 34, 9745–9753. [Google Scholar] [CrossRef]
- Zhuang, X.; Xia, X.; Xu, X.; Li, L. Experimental investigation on hydrogen production by methanol steam reforming in a novel multichannel micro packed bed reformer. Int. J. Hydrog. Energy 2020, 45, 11024–11034. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.; Constantinou, A. Computational fluid dynamics (CFD) and reaction modelling study of bio-oil catalytic hydrodeoxygenation in microreactors. React. Chem. Eng. 2020, 5, 1083–1092. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.; Constantinou, A. Modelling of packed bed and coated wall microreactors for methanol steam reforming for hydrogen production. RSC Adv. 2020, 10, 41680–41692. [Google Scholar] [CrossRef]
- Karim, A.; Bravo, J.; Gorm, D.; Conant, T.; Datye, A. Comparison of wall-coated and packed-bed reactors for steam reforming of methanol. Catal. Today 2005, 110, 86–91. [Google Scholar] [CrossRef]
- Caiti, M.; Padovan, D.; Hammond, C. Continuous production of hydrogen from formic acid decomposition over heterogeneous nanoparticle catalysts: From batch to continuous flow. ACS Catal. 2019, 9, 9188–9198. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Beloshapkin, S.; Ross, J.R. Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal. Today 2010, 154, 7–12. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Butt, J.B. Catalyst Deactivation 1991; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Fogler, H. Chapter 10: Catalysis and Catalytic Reactors. In Elements of Chemical Reaction Engineering, 5th ed.; Pearson: London, UK, 2016; pp. 454–456. [Google Scholar]
- Lv, Q.; Meng, Q.; Liu, W.; Sun, N.; Jiang, K.; Ma, L.; Peng, Z.; Cai, W.; Liu, C.; Ge, J.; et al. Pd–PdO interface as active site for HCOOH selective dehydrogenation at ambient condition. J. Phys. Chem. C 2018, 122, 2081–2088. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Cazorla-Amorós, D. Hydrogen production from formic acid attained by bimetallic heterogeneous PdAg catalytic systems. Energies 2019, 12, 4027. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qi, Y.; Zhang, D.; Liu, C. New insight into the decomposition mechanism of formic acid on Pd(111): Competing formation of CO2 and CO. J. Phys. Chem. C 2014, 118, 2067–2076. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Zahmakiran, M.; Kaya, M. Carbon supported trimetallic PdNiAg nanoparticles as highly active, selective and reusable catalyst in the formic acid decomposition. Appl. Catal. B Environ. 2014, 160, 514–524. [Google Scholar] [CrossRef]
- Peter, M.; Adamovsky, S.; Camacho, J.M.F.; Schauermann, S. Energetics of elementary reaction steps relevant for CO oxidation: CO and O2 adsorption on model Pd nanoparticles and Pd(111). Faraday Discuss. 2013, 162, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.-B. B-Doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Ruthven, D.; Upadhye, R. The catalytic decomposition of aqueous formic acid over suspended palladium catalysts. J. Catal. 1971, 21, 39–47. [Google Scholar] [CrossRef]
- Hu, C.; Pulleri, J.K.; Ting, S.-W.; Chan, K.-Y. Activity of Pd/C for hydrogen generation in aqueous formic acid solution. Int. J. Hydrog. Energy 2014, 39, 381–390. [Google Scholar] [CrossRef]
- Simakova, I.L.; Prosvirin, I.P.; Kriventsov, V.V.; Parmon, V.N. The effect of preparation conditions on the catalytic and physical-chemical properties of Pd/C in reductive debenzylation of hexabenzylhexaazaisowurtzitane. J. Sib. Fed. Univ. Chem. 2012, 5, 239–250. [Google Scholar]
- Troitskii, S.Y.; Nartova, A.; Moroz, E. Low-temperature carbonization of active centers is the reason of Pd/C catalysts deactivation. J. Struct. Chem. 2021, 62, 163–170. [Google Scholar] [CrossRef]
- Vinayakumar, K.; Palliyarayil, A.; Prakash, P.S.; Nandakumar, S.; Kumar, N.S.; Sil, S. Studies on the deactivation and activation of palladium impregnated carbon catalyst for environmental applications. Mater. Today Proc. 2020, 31, 631–639. [Google Scholar] [CrossRef]
- Cao, E.; Sankar, M.; Firth, S.; Lam, K.F.; Bethell, D.; Knight, D.K.; Hutchings, G.J.; McMillan, P.F.; Gavriilidis, A. Reaction and Raman spectroscopic studies of alcohol oxidation on gold-palladium catalysts in microstructured reactors. Chem. Eng. J. 2011, 167, 734–743. [Google Scholar] [CrossRef]
- Hu, C.; Ting, S.-W.; Tsui, J.; Chan, K.-Y. Formic acid dehydrogenation over PtRuBiOx/C catalyst for generation of CO-free hydrogen in a continuous-flow reactor. Int. J. Hydrogen Energy 2012, 37, 6372–6380. [Google Scholar] [CrossRef]
- Bueres, R.F.; Asedegbega-Nieto, E.; Díaz, E.; Ordóñez, S.; Díez, F.V. Performance of carbon nanofibres, high surface area graphites, and activated carbons as supports of Pd-based hydrodechlorination catalysts. Catal. Today 2010, 150, 16–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, L.; Medrano, J.A.; Ross, J.R.; Lefferts, L. Supported Pd catalysts prepared via colloidal method: The effect of acids. ACS Catal. 2013, 3, 2341–2352. [Google Scholar] [CrossRef]
- Reddy, K.; Doraiswamy, L. Estimating liquid diffusivity. Ind. Eng. Chem. Fundam. 1967, 6, 77–79. [Google Scholar] [CrossRef]














| Number of Elements | 406118 | 812236 | 1218354 |
|---|---|---|---|
| XHCOOH | 0.0912 | 0.0911 | 0.0931 |
| Symbol | Value | Units | Description |
|---|---|---|---|
| cFA | mol m−3 | Concentration of reacting gases | |
| y1 | m | Height of packed bed | |
| x1 | m | Catalyst loading length of packed bed | |
| mc | kg | Mass of catalyst | |
| υ | mL min−1 | Volumetric inlet flow rate | |
| T | 303–333 | K | Reaction temperature |
| dpe | nm | Size of catalyst pellet | |
| ε | 0.4 | - | Catalyst bed porosity |
| ρb | 1300 | kg m−3 | Catalyst density |
| R | 8.314 | J mol−1 K−1 | Ideal gas constant |
| DFA | m2 s−1 | Bulk fluid diffusion coefficient | |
| DFA,p | m2 s−1 | Effective diffusion coefficient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, S.; Sanchez, F.; Al-Salem, S.M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A. Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies. Catalysts 2021, 11, 341. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030341
Hafeez S, Sanchez F, Al-Salem SM, Villa A, Manos G, Dimitratos N, Constantinou A. Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies. Catalysts. 2021; 11(3):341. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030341
Chicago/Turabian StyleHafeez, Sanaa, Felipe Sanchez, Sultan M. Al-Salem, Alberto Villa, George Manos, Nikolaos Dimitratos, and Achilleas Constantinou. 2021. "Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies" Catalysts 11, no. 3: 341. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030341