Effect of Background Water Matrices on Pharmaceutical and Personal Care Product Removal by UV-LED/TiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption and Photolysis Control
2.2. Removal of PPCPs during Photocatalytic Treatment
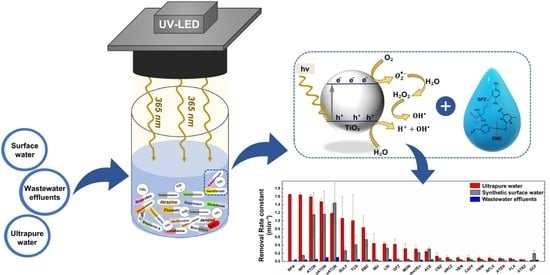
2.2.1. Effect of Water Matrices
2.2.2. Effect of Physicochemical Characteristics
2.3. Electrical Energy per Order Required for Removal
3. Materials and Methods
3.1. Water Matrices
3.2. Photocatalytic Degradation of PPCPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.L.; Xiong, Z. Pillaring Chemically Exfoliated Graphene Oxide with Carbon Nanotubes for Photocatalytic Degradation of Dyes under Visible Light Irradiation. ACS Nano 2010, 4, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, K.T.; Viswanathan, B. Synthesis, Characterization and Photocatalytic Properties of Iron-Doped TiO2 Catalysts. J. Photochem. Photobiol. A Chem. 1997, 108, 79–84. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of Residual Pharmaceuticals from Aqueous Systems by Advanced Oxidation Processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Liang, R.; Van Leuwen, J.C.; Bragg, L.M.; Arlos, M.J.; Fong, L.C.M.L.C.; Schneider, O.M.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P. Utilizing UV-LED Pulse Width Modulation on TiO2 Advanced Oxidation Processes to Enhance the Decomposition Efficiency of Pharmaceutical Micropollutants. Chem. Eng. J. 2019, 361, 439–449. [Google Scholar] [CrossRef]
- Fattahi, A.; Arlos, M.J.; Bragg, L.M.; Kowalczyk, S.; Liang, R.; Schneider, O.M.; Zhou, N.; Servos, M.R. Photodecomposition of Pharmaceuticals and Personal Care Products Using P25 Modified with Ag Nanoparticles in the Presence of Natural Organic Matter. Sci. Total Environ. 2020, 752, 142000. [Google Scholar] [CrossRef] [PubMed]
- Arlos, M.J.; Liang, R.; Hatat-Fraile, M.M.; Bragg, L.M.; Zhou, N.Y.; Servos, M.R.; Andrews, S.A. Photocatalytic Decomposition of Selected Estrogens and Their Estrogenic Activity by UV-LED Irradiated TiO2 Immobilized on Porous Titanium Sheets via Thermal-Chemical Oxidation. J. Hazard. Mater. 2016, 318, 541–550. [Google Scholar] [CrossRef]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic Decomposition of Organic Micropollutants Using Immobilized TiO2 Having Different Isoelectric Points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef]
- Arlos, M.J.; Liang, R.; Fong, L.C.M.; Zhou, N.Y.; Ptacek, C.J.; Andrews, S.A.; Servos, M.R. Influence of Methanol When Used as a Water-Miscible Carrier of Pharmaceuticals in TiO2 Photocatalytic Degradation Experiments. J. Environ. Chem. Eng. 2017, 5, 4497–4504. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Choi, Y.; Kim, S.; Lee, S.; Lee, S.; Choi, W.; Lee, J. Heterogeneous Photocatalytic Treatment of Pharmaceutical Micropollutants: Effects of Wastewater Effluent Matrix and Catalyst Modifications. Appl. Catal. B Environ. 2014, 147, 8–16. [Google Scholar] [CrossRef]
- Autin, O.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. The Impact of Background Organic Matter and Alkalinity on the Degradation of the Pesticide Metaldehyde by Two Advanced Oxidation Processes: UV/H2O2 and UV/TiO2. Water Res. 2013, 47, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Zepp, R.G.; Baughman, G.L.; Schlotzhauer, P.F. Comparison of Photochemical Behavior of Various Humic Substances in Water: I. Sunlight Induced Reactions of Aquatic Pollutants Photosensitized by Humic Substances. Chemosphere 1981, 10, 109–117. [Google Scholar] [CrossRef]
- Pereira, V.J.; Linden, K.G.; Weinberg, H.S. Evaluation of UV Irradiation for Photolytic and Oxidative Degradation of Pharmaceutical Compounds in Water. Water Res. 2007, 41, 4413–4423. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Haroune, L.; Salaun, M.; Ménard, A.; Legault, C.Y.; Bellenger, J.-P. Photocatalytic Degradation of Carbamazepine and Three Derivatives Using TiO2 and ZnO: Effect of PH, Ionic Strength, and Natural Organic Matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.-M. Why Inorganic Salts Decrease the TiO2 Photocatalytic Efficiency. Int. J. Photoenergy 2005, 7, 641208. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant Sorption to Membrane Polymers: A Review of Mechanisms for Estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef]
- Liang, R.; Li Chun Fong, L.C.M.; Arlos, M.J.; Van Leeuwen, J.; Shahnam, E.; Peng, P.; Servos, M.R.; Zhou, Y.N. Photocatalytic Degradation Using One-Dimensional TiO2 and Ag-TiO2 Nanobelts under UV-LED Controlled Periodic Illumination. J. Environ. Chem. Eng. 2017, 5, 4365–4373. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Beltrán, F.J. TiO2 Photocatalytic Oxidation of a Mixture of Emerging Contaminants: A Kinetic Study Independent of Radiation Absorption Based on the Direct-Indirect Model. Chem. Eng. J. 2018, 339, 369–380. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of Reactive Oxygen Species on the Activity of Noble Metal-Doped TiO2 Photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.; Robert, D.; Weber, J.V. Influence of PH and Chloride Anion on the Photocatalytic Degradation of Organic Compounds: Part I. Effect on the Benzamide and Para-Hydroxybenzoic Acid in TiO2 Aqueous Solution. Appl. Catal. B Environ. 2001, 35, 117–124. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.V. Photocatalytic Mineralization of Humic Acids with TiO2: Effect of PH, Sulfate and Chloride Anions. Int. J. Photoenergy 2003, 5, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.; Low, G.K.C.; Matthews, R.W. Effects of Common Inorganic Anions on Rates of Photocatalytic Oxidation of Organic Carbon over Illuminated Titanium Dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zahraa, O.; Bouchy, M. Inhibition of the Adsorption and Photocatalytic Degradation of an Organic Contaminant in an Aqueous Suspension of TiO2 by Inorganic Ions. J. Photochem. Photobiol. A Chem. 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Wang, K.-H.; Hsieh, Y.-H.; Wu, C.-H.; Chang, C.-Y. The PH and Anion Effects on the Heterogeneous Photocatalytic Degradation of O-Methylbenzoic Acid in TiO2 Aqueous Suspension. Chemosphere 2000, 40, 389–394. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Washington, DC, USA, 1985; Volume 2254. [Google Scholar]
- Brezonik, P.L.; Fulkerson-Brekken, J. Nitrate-Induced Photolysis in Natural Waters: Controls on Concentrations of Hydroxyl Radical Photo-Intermediates by Natural Scavenging Agents. Environ. Sci. Technol. 1998, 32, 3004–3010. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Wender, H.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A., Jr. Photocatalytic Treatment of Metoprolol with B-Doped TiO2: Effect of Water Matrix, Toxicological Evaluation and Identification of Intermediates. Appl. Catal. B Environ. 2015, 176, 173–182. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical Degradation of Oxytetracycline: Influence of PH and Role of Carbonate Radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Lai, W.W.-P.; Hsu, M.-H.; Lin, A.Y.-C. The Role of Bicarbonate Anions in Methotrexate Degradation via UV/TiO2: Mechanisms, Reactivity and Increased Toxicity. Water Res. 2017, 112, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of Sulfamethoxazole and Related Antimicrobial Agents by TiO2 Photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef] [PubMed]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer Method to Determine Rate Constants for the Reaction of Carbonate Radical with Organic Compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Zhang, G.; He, X.; Nadagouda, M.N.; O’Shea, K.E.; Dionysiou, D.D. The Effect of Basic PH and Carbonate Ion on the Mechanism of Photocatalytic Destruction of Cylindrospermopsin. Water Res. 2015, 73, 353–361. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Drikas, M.; Amal, R. TiO2 Photocatalysis of Natural Organic Matter in Surface Water: Impact on Trihalomethane and Haloacetic Acid Formation Potential. Environ. Sci. Technol. 2008, 42, 6218–6223. [Google Scholar] [CrossRef] [PubMed]
- Ndlangamandla, N.G.; Kuvarega, A.T.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. A Novel Photodegradation Approach for the Efficient Removal of Natural Organic Matter (NOM) from Water. Phys. Chem. Earth 2018, 106, 97–106. [Google Scholar] [CrossRef]
- Gora, S.; Sokolowski, A.; Hatat-Fraile, M.; Liang, R.; Zhou, Y.N.; Andrews, S. Solar Photocatalysis with Modified TiO2 Photocatalysts: Effects on NOM and Disinfection Byproduct Formation Potential. Environ. Sci. Water Res. Technol. 2018, 4, 1361–1376. [Google Scholar] [CrossRef]
- Garbin, J.R.; Milori, D.M.B.P.; Simoes, M.L.; Da Silva, W.T.L.; Neto, L.M. Influence of Humic Substances on the Photolysis of Aqueous Pesticide Residues. Chemosphere 2007, 66, 1692–1698. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Fate of Pharmaceuticals—Photodegradation by Simulated Solar UV-Light. Chemosphere 2003, 52, 1757–1769. [Google Scholar] [CrossRef]
- Carlos, L.; Mártire, D.O.; Gonzalez, M.C.; Gomis, J.; Bernabeu, A.; Amat, A.M.; Arques, A. Photochemical Fate of a Mixture of Emerging Pollutants in the Presence of Humic Substances. Water Res. 2012, 46, 4732–4740. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-Immobilized Electrospun TiO2 Nanofibers for Photocatalytic Degradation of Pharmaceuticals: The Effects of PH and Dissolved Organic Matter Characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Ku, Y.; Irawan, A. Photodecomposition of O-Cresol by UV-LED/TiO2 Process with Controlled Periodic Illumination. Chemosphere 2007, 69, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.; Arlos, M.J.; Bragg, L.M.; Liang, R.; Zhou, N.; Servos, M.R. Degradation of Natural Organic Matter Using Ag-P25 Photocatalyst under Continuous and Periodic Irradiation of 405 and 365 Nm UV-LEDs. J. Environ. Chem. Eng. 2021, 9, 104844. [Google Scholar] [CrossRef]
- Lamsal, R.; Walsh, M.E.; Gagnon, G.A. Comparison of Advanced Oxidation Processes for the Removal of Natural Organic Matter. Water Res. 2011, 45, 3263–3269. [Google Scholar] [CrossRef]
- Madras, G. Kinetics of Simultaneous Photocatalytic Degradation of Phenolic Compounds and Reduction of Metal Ions with Nano-TiO2. Environ. Sci. Technol. 2008, 42, 913–919. [Google Scholar]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of Membrane Filtration and Advanced Oxidation Processes for Removal of Pharmaceutical Residues: A Critical Review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Lu, M. Photocatalysis and Water Purification: From Fundamentals to Recent Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hu, A.; Zhang, X.; Luong, D.; Oakes, K.D.; Servos, M.R.; Liang, R.; Kurdi, S.; Peng, P.; Zhou, Y. Adsorption and Photocatalytic Degradation Kinetics of Pharmaceuticals by TiO2 Nanowires during Water Treatment. Waste Biomass Valoriz. 2012, 3, 443–449. [Google Scholar] [CrossRef]
- Kim, I.; Tanaka, H. Photodegradation Characteristics of PPCPs in Water with UV Treatment. Environ. Int. 2009, 35, 793–802. [Google Scholar] [CrossRef]
- Fernández, R.L.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of Pharmaceuticals and Endocrine Disrupting Chemicals by a Submerged Membrane Photocatalysis Reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Cater, S.R.; Stefan, M.I.; Bolton, J.R.; Safarzadeh-Amiri, A. UV/H2O2 Treatment of Methyl Tert-Butyl Ether in Contaminated Waters. Environ. Sci. Technol. 2000, 34, 659–662. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Patil, M.K.; Nadagouda, M.N.; Dionysiou, D.D. Hydrothermal Synthesis of Photoactive Nitrogen-and Boron-Codoped TiO2 Nanoparticles for the Treatment of Bisphenol A in Wastewater: Synthesis, Photocatalytic Activity, Degradation Byproducts and Reaction Pathways. Appl. Catal. B Environ. 2019, 241, 598–611. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of Immobilized TiO2 on PVDF Dual Layer Hollow Fibre Membrane to Improve the Photocatalytic Removal of Pharmaceuticals in Different Water Matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic Treatment of Organic Pollutants in a Synthetic Wastewater Using UV Light and Combinations of TiO2, H2O2 and Fe (III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef]





| Parameters | Units | Synthetic Surface Water | Wastewater Effluent |
|---|---|---|---|
| pH | 8–8.5 | 7–7.5 | |
| TOC | mg L−1 | 6.12 | 9.37 |
| Dissolved Chloride | mg L−1 | 40 | 490 |
| Hardness | mg L−1 as CaCO3 | 114 | N/A |
| Alkalinity | mg L−1 as CaCO3 | 117 | N/A |
| Nitrate | mg L−1 | 3 | 36.53 |
| Nitrite | mg L−1 | N/A | 0.53 |
| Sulfate | mg L−1 | 241 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattahi, A.; Jaciw-Zurakowsky, I.; Srikanthan, N.; Bragg, L.; Liang, R.; Zhou, N.; Servos, M.; Arlos, M. Effect of Background Water Matrices on Pharmaceutical and Personal Care Product Removal by UV-LED/TiO2. Catalysts 2021, 11, 576. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050576
Fattahi A, Jaciw-Zurakowsky I, Srikanthan N, Bragg L, Liang R, Zhou N, Servos M, Arlos M. Effect of Background Water Matrices on Pharmaceutical and Personal Care Product Removal by UV-LED/TiO2. Catalysts. 2021; 11(5):576. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050576
Chicago/Turabian StyleFattahi, Azar, Ivana Jaciw-Zurakowsky, Nivetha Srikanthan, Leslie Bragg, Robert Liang, Norman Zhou, Mark Servos, and Maricor Arlos. 2021. "Effect of Background Water Matrices on Pharmaceutical and Personal Care Product Removal by UV-LED/TiO2" Catalysts 11, no. 5: 576. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050576