An Investigation into the Bulk and Surface Phase Transformations of Bimetallic Pd-In/Al2O3 Catalyst during Reductive and Oxidative Treatments In Situ
Abstract
:1. Introduction
2. Results and Discussion
2.1. TEM Characterization
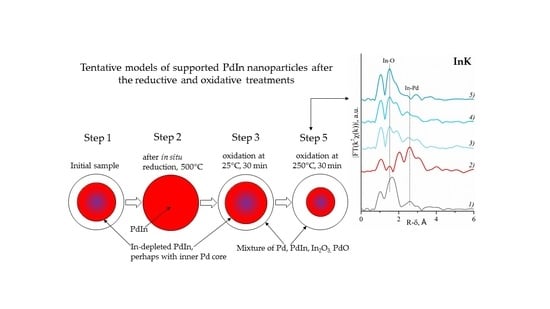
2.2. EXAFS and XANES Spectroscopy In Situ
2.2.1. Palladium Extended X-ray Absorption Fine Structure
2.2.2. Indium Extended X-ray Absorption Fine Structure
2.2.3. XANES Linear Combination Fit
2.3. DRIFT Spectroscopy of Adsorbed CO
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Transmission Electron Microscopy
3.3. In Situ XAFS-Spectroscopy
3.4. DRIFT Spectroscopy of Adsorbed CO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dhiman, M.; Polshettiwar, V. Supported Single Atom and Pseudo-Single Atom of Metals as Sustainable Heterogeneous Nanocatalysts. ChemCatChem 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Qin, R.; Liu, P.; Fu, G.; Zheng, N. Strategies for Stabilizing Atomically Dispersed Metal Catalysts. Small Methods 2018, 2, 1700286. [Google Scholar] [CrossRef]
- Bauer, J.C.; Chen, X.; Liu, Q.; Phan, T.H.; Schaak, R.E. Converting nanocrystalline metals into alloys and intermetallic compounds for applications in catalysis. J. Mater. Chem. 2008, 18, 275–282. [Google Scholar] [CrossRef]
- Xiao, W.; Lei, W.; Gong, M.; Xin, H.L.; Wang, D. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 2018, 8, 3237–3256. [Google Scholar] [CrossRef]
- Williams, B.P.; Qi, Z.; Huang, W.; Tsung, C.K. The impact of synthetic method on the catalytic application of intermetallic nanoparticles. Nanoscale 2020, 12, 18545–18562. [Google Scholar] [CrossRef]
- Li, J.; Sun, S. Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef]
- Ma, T.; Wang, S.; Chen, M.; Maligal-Ganesh, R.V.; Wang, L.L.; Johnson, D.D.; Kramer, M.J.; Huang, W.; Zhou, L. Toward phase and catalysis control: Tracking the formation of intermetallic nanoparticles at atomic scale. Chem 2019, 5, 1235–1247. [Google Scholar] [CrossRef] [Green Version]
- Gamler, J.T.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random alloyed versus intermetallic nanoparticles: A comparison of electrocatalytic performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef]
- Penner, S.; Armbrüster, M. Formation of intermetallic compounds by reactive metal–support interaction: A frequently encountered phenomenon in catalysis. ChemCatChem 2015, 7, 374–392. [Google Scholar] [CrossRef]
- Armbruster, M.; Schlögl, R.; Grin, Y. Intermetallic compounds in heterogeneous catalysis—A quickly developing field. Sci. Technol. Adv. Mater. 2014, 15, 034803. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ma, Z.; Wu, Q.; Cai, Y.; Huang, Y.; Liu, K.; Fan, Y.; Wang, H.; Li, Q.; Qi, J.; et al. Promoting the ORR catalysis of Pt-Fe intermetallic catalysts by increasing atomic utilization and electronic regulation. Electrochim. Acta 2020, 330, 135119. [Google Scholar] [CrossRef]
- Li, J.; Xi, Z.; Pan, Y.T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 2018, 140, 2926–2932. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Kang, Y.; Xia, D.; Gan, L. Rational development of structurally ordered platinum ternary intermetallic electrocatalysts for oxygen reduction reaction. Catalysts 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Miura, A.; Wang, H.; Leonard, B.M.; Abruna, H.D.; DiSalvo, F.J. Synthesis of intermetallic PtZn nanoparticles by reaction of Pt nanoparticles with Zn vapor and their application as fuel cell catalysts. Chem. Mater. 2009, 21, 2661–2667. [Google Scholar] [CrossRef]
- Qi, Z.; Xiao, C.; Liu, C.; Goh, T.W.; Zhou, L.; Maligal-Ganesh, R.; Pei, Y.; Li, X.; Curtiss, L.; Huang, W. Sub-4 nm PtZn intermetallic nanoparticles for enhanced mass and specific activities in catalytic electrooxidation reaction. J. Am. Chem. Soc. 2017, 139, 4762–4768. [Google Scholar] [CrossRef] [Green Version]
- Cesar, L.G.; Yang, C.; Lu, Z.; Ren, Y.; Zhang, G.; Miller, J.T. Identification of a Pt3Co surface intermetallic alloy in Pt–Co propane dehydrogenation catalysts. ACS Catal. 2019, 9, 5231–5244. [Google Scholar] [CrossRef]
- Wegener, E.C.; Wu, Z.; Tseng, H.T.; Gallagher, J.R.; Ren, Y.; Diaz, R.E.; Ribeiro, F.H.; Miller, J.T. Structure and reactivity of Pt–In intermetallic alloy nanoparticles: Highly selective catalysts for ethane dehydrogenation. Catal. Today 2018, 299, 146–153. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, W.I.; Kim, J.R.; Oh, K.; Koh, H.L. Effect of direct reduction treatment on Pt–Sn/Al2O3 catalyst for propane dehydrogenation. Catalysts 2019, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Fiordaliso, E.M.; Sharafutdinov, I.; Carvalho, H.W.; Grunwaldt, J.D.; Hansen, T.W.; Chorkendorff, I.; Wagner, J.; Damsgaard, C.D. Intermetallic GaPd2 nanoparticles on SiO2 for low-pressure CO2 hydrogenation to methanol: Catalytic performance and in situ characterization. ACS Catal. 2015, 5, 5827–5836. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.; Williams, C.K. PdIn intermetallic nanoparticles for the hydrogenation of CO2 to methanol. Appl. Catal. B 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Ota, A.; Kunkes, E.L.; Kasatkin, I.; Groppo, E.; Ferri, D.; Poceiro, B.; Navarro Yegra, R.M.; Behrens, M. Comparative study of hydrotalcite-derived supported Pd2Ga and PdZn intermetallic nanoparticles as methanol synthesis and methanol steam reforming catalysts. J. Catal. 2012, 293, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A comparative discussion of the catalytic activity and CO2-Selectivity of Cu-Zr and Pd-Zr (intermetallic) compounds in methanol steam reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, M. Intermetallic compounds in catalysis—A versatile class of materials meets interesting challenges. Sci. Tech. Adv. Mater. 2020, 21, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Snider, J.L.; Streibel, V.; Hubert, M.A.; Choksi, T.S.; Valle, E.; Upham, D.C.; Schumann, J.; Duyar, M.S.; Gallo, A.; Abild-Pedersen, F.; et al. Revealing the Synergy between Oxide and Alloy Phases on the Performance of Bimetallic In−Pd Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 3399–3412. [Google Scholar] [CrossRef]
- Lorenz, H.; Turner, S.; Lebedev, O.I.; Tendeloo, G.V.; Klotzer, B.; Rameshan, C.; Pfaller, K.; Penner, S. Pd–In2O3 interaction due to reduction in hydrogen: Consequences for methanol steam reforming. Appl. Catal. A Gen. 2010, 374, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wegener, E.C.; Tseng, H.-T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E.; Ren, Y.; Ribeiroa, F.H.; Miller, J.T. Pd–In intermetallic alloy nanoparticles: Highly selective ethane dehydrogenation catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Cao, Y.; Sui, Z.; Zhu, Y.; Zhou, X.; Chen, D. Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-Dependent Performance. ACS Catal. 2017, 7, 7835–7846. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, Y.-R.; Chiang, C.-H.; Tung, K.-L.; Yeh, T.-K.; Tuan, H.-Y. Monodisperse ordered indium–palladium nanoparticles: Synthesis and role of indium for boosting superior electrocatalytic activity for ethanol oxidation reaction. Nanoscale 2019, 11, 3336–3343. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Irusta, S.; Querini, C.; Miro, E. Spectroscopic and catalytic characterization of Pd–In and Pt–In supported on Al2O3 and SiO2, active catalysts for nitrate hydrogenation. Appl. Catal. A Gen. 2008, 348, 60–70. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Fang, D. Effect of indium-modified palladium catalysts on the hydrodechlorination of 4-chlorophenol. RSC Adv. 2015, 5, 42861–42868. [Google Scholar] [CrossRef]
- Knight, J.R.; Rhys, D.W. The systems palladium-indium and palladium-tin. J. Less Common Met. 1959, 1, 292–303. [Google Scholar] [CrossRef]
- Markov, P.V.; Smirnova, N.S.; Baeva, G.N.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Intermetallic PdxIny/Al2O3 catalysts with isolated single-atom Pd sites for one-pot hydrogenation of diphenylacetylene into trans-stilbene. Mendeleev Commun. 2020, 30, 468–471. [Google Scholar] [CrossRef]
- Markov, P.V.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Smirnova, N.S.; Prosvirin, I.P.; Vinokurov, Z.S.; Panafidin, M.A.; Baeva, G.N.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. PdIn/Al2O3 Intermetallic Catalyst: Structure and Catalytic Characteristics in Selective Hydrogenation of Acetylene. Kinet. Catal. 2019, 60, 842–850. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Smirnova, N.S.; Krivoruchenko, D.S.; Baeva, G.N.; Mashkovsky, I.S.; Yakushev, I.A.; Vargaftik, M.N. Single-atom Pd sites on the surface of Pd–In nanoparticles supported on γ-Al2O3: A CO-DRIFTS study. Mendeleev Commun. 2017, 27, 515–517. [Google Scholar] [CrossRef]
- Furukawa, S.; Endo, M.; Komatsu, T. Bifunctional Catalytic System Effective for Oxidative Dehydrogenation of 1-Butene and n-Butane Using Pd-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3533–3542. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Peter, S.C. Synthetically tuned electronic and geometrical properties of intermetallic compounds as effective heterogeneous catalysts. Prog. Solid State Chem. 2018, 52, 1–30. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Lopez-Sanchez, J.A.; Jackson, S.D.; Klapötke, T.M.; Bäumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The application of infrared spectroscopy to probe the surface morphology of alumina supported palladium catalysts. J. Chem. Phys. 2006, 124, 174706. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Bragina, G.O.; Baeva, G.N.; Smirnova, N.S.; Kazakov, A.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. Formation of Isolated Single-Atom Pd1 Sites on the Surface of Pd–Ag/Al2O3 Bimetallic Catalysts. Kinet. Catal. 2020, 61, 758–767. [Google Scholar] [CrossRef]
- Trofimova, N.N.; Veligzhanin, A.A.; Murzin, V.Y.; Chernyshov, A.A.; Khramov, E.V.; Zabluda, V.N.; Edel’man, I.S.; Slovokhotov, Y.L.; Zubavichus, Y.V. Structural diagnostics of functional nanomaterials with the use of X-ray synchrotron radiation. Nanotechnol. Russ. 2013, 8, 396–401. [Google Scholar] [CrossRef]
- Veligzhanin, A.A.; Zubavichus, Y.V.; Chernyshov, A.A.; Trigub, A.L.; Khlebnikov, A.S.; Nizovskii, A.I.; Bukhtiyarov, V.I. An in situ cell for investigation of the catalyst structure using synchrotron radiation. J. Struct. Chem. 2010, 51, 20–27. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- OriginLab. Available online: https://www.originlab.com/ (accessed on 23 June 2021).




| X-ray Absorption Edge | Scattering Pathway | Initial Sample | H2/He, 500 °C, 1 h | O2/N2, 25 °C, 30 min | O2/N2, 100 °C, 30 min | O2/N2, 250 °C, 30 min |
|---|---|---|---|---|---|---|
| Pd K | In | 7.4 | 6.3 | 5.6 | 7.9 | 7.9 |
| In K | O | 5.4 | - | 2.8 | 3.8 | 4.6 |
| Pd | 3.9 | 4.7 | 3.0 | 4.4 | 1.4 | |
| In | - | 2.4 | 2.1 | 1.0 | 1.4 |
| Step | Pd K XANES Linear Fit | In K XANES Linear Fit | ||
|---|---|---|---|---|
| Pd in PdIn, at.% | Pd in Monometallic Pd, at.% | In in PdIn, at.% | In in In2O3, at.% | |
| initial sample | 48 | 52 | 35 | 65 |
| reduced 500 °C | 100 1 | - | 100 1 | - |
| oxidized 25 °C | 79 | 21 | 60 | 40 |
| oxidized 100 °C | 74 | 26 | 50 | 50 |
| oxidized 250 °C | 55 | 45 | 34 | 66 |
| Step | Pd in PdIn, at.% | Pd in Monometallic Pd, at.% |
|---|---|---|
| initial sample | 49 | 51 |
| reduced 500 °C | 100 | - |
| oxidized 25 °C | 71 | 29 |
| oxidized 100 °C | 51 | 49 |
| oxidized 250 °C | 38 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, N.S.; Khramov, E.V.; Baeva, G.N.; Markov, P.V.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. An Investigation into the Bulk and Surface Phase Transformations of Bimetallic Pd-In/Al2O3 Catalyst during Reductive and Oxidative Treatments In Situ. Catalysts 2021, 11, 859. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070859
Smirnova NS, Khramov EV, Baeva GN, Markov PV, Bukhtiyarov AV, Zubavichus YV, Stakheev AY. An Investigation into the Bulk and Surface Phase Transformations of Bimetallic Pd-In/Al2O3 Catalyst during Reductive and Oxidative Treatments In Situ. Catalysts. 2021; 11(7):859. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070859
Chicago/Turabian StyleSmirnova, Nadezhda S., Evgeny V. Khramov, Galina N. Baeva, Pavel V. Markov, Andrey V. Bukhtiyarov, Yan V. Zubavichus, and Aleksander Y. Stakheev. 2021. "An Investigation into the Bulk and Surface Phase Transformations of Bimetallic Pd-In/Al2O3 Catalyst during Reductive and Oxidative Treatments In Situ" Catalysts 11, no. 7: 859. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070859