Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation
Abstract
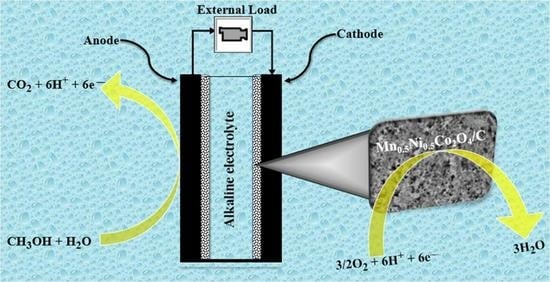
:1. Introduction
2. Results and Discussions
2.1. Spinel Synthesis Chemistry
2.2. Physicochemical Characterization
2.3. Electrochemical Evaluation of ORR Activity
3. Materials and Methods
3.1. Synthesis of MnxNi1−xCo2O4
3.2. Synthesis of Porous Mn0.5Ni0.5Co2O4/C Nanosheets
3.3. Physicochemical Characterizations
3.4. Preparation and Electrochemical Characterization of Modified Electrodes
3.4.1. Preparation of Modified Electrodes
3.4.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Ho, J.H.; Li, Y.; Dai, Y.; Kim, T.I.; Wang, J.; Ren, J.; Yun, H.S.; Liu, X. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2021, 46, 20503–20515. [Google Scholar] [CrossRef]
- Gwebu, S.S.; Nomngongo, P.N.; Maxakato, N.W. Pt/CNDs-TiO2 electrocatalyst for direct alcohol fuel cells. Mater. Today Proc. 2018, 5, 10460–10469. [Google Scholar] [CrossRef]
- Mohammed, H.; Al-Othman, A.; Nancarrow, P.; Tawalbeh, M.; El Haj Assad, M. Direct hydrocarbon fuel cells: A promising technology for improving energy efficiency. Energy 2019, 172, 207–219. [Google Scholar] [CrossRef]
- Cermenek, B.; Ranninger, J.; Hacker, V. Alkaline Direct Ethanol Fuel Cell; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 1904, pp. 383–405. [Google Scholar]
- Boppella, R.; Lee, J.E.; Mota, F.M.; Kim, J.Y.; Feng, Z.; Kim, D.H. Composite hollow nanostructures composed of carbon-coated Ti3+ self-doped TiO2-reduced graphene oxide as an efficient electrocatalyst for oxygen reduction. J. Mater. Chem. A 2017, 5, 7072–7080. [Google Scholar] [CrossRef]
- Majlan, E.H.; Rohendi, D.; Daud, W.R.W.; Husaini, T.; Haque, M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.I.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Ramaprabhu, S. Platinum-TM (TM = Fe, Co) alloy nanoparticles dispersed nitrogen doped (reduced graphene oxide-multiwalled carbon nanotube) hybrid structure cathode electrocatalysts for high performance PEMFC applications. Nanoscale 2013, 5, 5109–5118. [Google Scholar] [CrossRef]
- Roudbari, M.N.; Ojani, R.; Raoof, J.B. Nitrogen functionalized carbon nanotubes as a support of platinum electrocatalysts for performance improvement of ORR using fuel cell cathodic half-cell. Renew. Energy 2020, 159, 1015–1028. [Google Scholar] [CrossRef]
- Shahbazi Farahani, F.; Mecheri, B.; Reza Majidi, M.; Costa de Oliveira, M.A.; D’Epifanio, A.; Zurlo, F.; Placidi, E.; Arciprete, F.; Licoccia, S. MnOx-based electrocatalysts for enhanced oxygen reduction in microbial fuel cell air cathodes. J. Power Sources 2018, 390, 45–53. [Google Scholar] [CrossRef]
- Dolla, T.H.; Pruessner, K.; Billing, D.G.; Sheppard, C.; Prinsloo, A.; Carleschi, E.; Doyle, B.; Ndungu, P. Sol-gel synthesis of MnxNi1−xCo2O4 spinel phase materials: Structural, electronic, and magnetic properties. J. Alloy. Compd. 2018, 742, 78–89. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Mardare, C.C. Preparation of Spinel Oxide Layers for High Temperature Fuel Cell Applications. Ph.D. Thesis, University Library Bochum, Bochum, Germany, 2009; p. 130. [Google Scholar]
- Thekkoot, S.R. Nanostructured Mixed Transition Metal Spinel Oxide as Efficient Electrocatalysts. Master’s Thesis, Faculty of Graduate Studies, Toronto, ON, Canada, 2015. [Google Scholar]
- Adeela, N.; Khan, U.; Naz, S.; Khan, K.; Sagar, R.U.R.; Aslam, S.; Wu, D. Role of Ni concentration on structural and magnetic properties of inverse spinel Ferrite. Mater. Res. Bull. 2018, 107, 60–65. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, J.H. A cobalt hydroxide nanosheet-mediated synthesis of core-shell-type Mn0.005Co2.995O4 spinel nanocubes as efficient oxygen electrocatalysts. Dalt. Trans. 2020, 49, 1652–1659. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X.Y. Mesoporous NiCo2O4 Nanoplates on Three-Dimensional Graphene Foam as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 28274–28282. [Google Scholar] [CrossRef]
- Huang, Q.; Li, W.; Lin, Q.; Zheng, X.; Pan, H.; Pi, D.; Shao, C.; Hu, C.; Zhang, H. Catalytic performance of Pd–NiCo2O4/SiO2 in lean methane combustion at low temperature. J. Energy Inst. 2018, 91, 733–742. [Google Scholar] [CrossRef]
- Béjar, J.; Álvarez‒Contreras, L.; Espinosa‒Magaña, F.; Ledesma‒García, J.; Arjona, N.; Arriaga, L.G. Zn‒air battery operated with a 3DOM trimetallic spinel (Mn0.5Ni0.5Co2O4) as the oxygen electrode. Electrochim. Acta 2021, 391, 138900. [Google Scholar] [CrossRef]
- Serov, A.; Andersen, N.I.; Roy, A.J.; Matanovic, I.; Artyushkova, K.; Atanassov, P. CuCo2O4 ORR/OER Bi-Functional Catalyst: Influence of Synthetic Approach on Performance. J. Electrochem. Soc. 2015, 162, F449–F454. [Google Scholar] [CrossRef]
- Béjar, J.; Espinosa-Magaña, F.; Guerra-Balcázar, M.; Ledesma-García, J.; Álvarez-Contreras, L.; Arjona, N.; Arriaga, L.G. Three-Dimensional-Order Macroporous AB2O4Spinels (A, B = Co and Mn) as Electrodes in Zn-Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 53760–53773. [Google Scholar] [CrossRef]
- Merabet, L.; Rida, K.; Boukmouche, N. Sol-gel synthesis, characterization, and supercapacitor applications of MCo2O4 (M = Ni, Mn, Cu, Zn) cobaltite spinels. Ceram. Int. 2018, 44, 11265–11273. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Huang, Z.; Zhu, W.; Zhao, Z.J.; Gong, J. Facet-evolution growth of Mn3O4@CoxMn3-xO4 electrocatalysts on Ni foam towards efficient oxygen evolution reaction. J. Catal. 2019, 369, 105–110. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Shi, F.; Zhang, Y.; Zhang, J.; Gu, C.; Wang, X.; Tu, J. Spinel manganese-nickel-cobalt ternary oxide nanowire array for high-performance electrochemical capacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 18040–18047. [Google Scholar] [CrossRef]
- He, L.; Ling, Z.Y.; Wu, M.Y.; Zhang, G.; Liu, S.Z.; Zhang, S.Q.; Ling, D.X. Thermal and humidity sensing behaviors of Mn1.85Co0.3Ni0.85 O4 thin films: Effects of adjusting the surface morphology. Appl. Surf. Sci. 2017, 410, 201–205. [Google Scholar] [CrossRef]
- Mhin, S.; Han, H.; Kim, K.M.; Lim, J.; Kim, D.; Lee, J.I.; Ryu, J.H. Synthesis of (Ni, Mn, Co) O4 nanopowder with single cubic spinel phase via combustion method. Ceram. Int. 2016, 42, 13654–13658. [Google Scholar] [CrossRef]
- Chang, S.K.; Lee, K.T.; Zainal, Z.; Tan, K.B.; Yusof, N.A.; Yusoff, W.M.D.W.; Lee, J.F.; Wu, N.L. Structural and electrochemical properties of manganese substituted nickel cobaltite for supercapacitor application. Electrochim. Acta 2012, 67, 67–72. [Google Scholar] [CrossRef]
- Stella, C.; Soundararajan, N.; Ramachandran, K. Structural, optical, and magnetic properties of Mn and Fe-doped Co3O4 nanoparticles. AIP Adv. 2015, 5, 087104. [Google Scholar] [CrossRef]
- Tamboli, M.S.; Dubal, D.P.; Patil, S.S.; Shaikh, A.F.; Deonikar, V.G.; Kulkarni, M.V.; Maldar, N.N.; Inamuddin; Asiri, A.M.; Gomez-Romero, P.; et al. Mimics of microstructures of Ni substituted Mn1−xNixCo2O4 for high energy density asymmetric capacitors. Chem. Eng. J. 2017, 307, 300–310. [Google Scholar] [CrossRef]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Rugged High-Entropy Alloy Nanowires with in Situ Formed Surface Spinel Oxide As Highly Stable Electrocatalyst in Zn–Air Batteries. ACS Mater. Lett. 2020, 2, 1698–1706. [Google Scholar] [CrossRef]
- Dolla, T.H.; Billing, D.G.; Sheppard, C.; Prinsloo, A.; Carleschi, E.; Doyle, B.P.; Pruessner, K.; Ndungu, P. Mn substituted MnxZn1−xCo2O4 oxides synthesized by co-precipitation; effect of doping on the structural, electronic and magnetic properties. RSC Adv. 2018, 8, 39837–39848. [Google Scholar] [CrossRef] [Green Version]
- Nestola, F.; Smyth, J.R.; Parisatto, M.; Secco, L.; Princivalle, F.; Bruno, M.; Prencipe, M.; Dal Negro, A. Effects of non-stoichiometry on the spinel structure at high pressure: Implications for Earth’s mantle mineralogy. Geochim. Cosmochim. Acta 2009, 73, 489–492. [Google Scholar] [CrossRef]
- De Koninck, M.; Marsan, B. MnxCu1−xCo2O4 used as bifunctional electrocatalyst in alkaline medium. Electrochim. Acta 2008, 53, 7012–7021. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 981–988. [Google Scholar] [CrossRef]
- Mondal, A.K.; Su, D.; Chen, S.; Ung, A.; Kim, H.S.; Wang, G. Mesoporous MnCo2O4with a flake-like structure as advanced electrode materials for lithium-ion batteries and supercapacitors. Chem. A Eur. J. 2015, 21, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Bitla, Y.; Chin, Y.Y.; Lin, J.C.; Van, C.N.; Liu, R.; Zhu, Y.; Liu, H.J.; Zhan, Q.; Lin, H.J.; Chen, C.T.; et al. Origin of metallic behavior in NiCo2O4 ferrimagnet. Sci. Rep. 2015, 5, 15201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. Facile synthesis of NiCoMnO4 nanoparticles as novel electrode materials for high-performance asymmetric energy storage devices. RSC Adv. 2016, 6, 28970–28980. [Google Scholar] [CrossRef]
- McNulty, D.; Geaney, H.; O’Dwyer, C. Carbon-Coated Honeycomb Ni-Mn-Co-O Inverse Opal: A High Capacity Ternary Transition Metal Oxide Anode for Li-ion Batteries. Sci. Rep. 2017, 7, 42263. [Google Scholar] [CrossRef] [PubMed]
- Charreteur, F.; Jaouen, F.; Ruggeri, S.; Dodelet, J.P. Fe/N/C non-precious catalysts for PEM fuel cells: Influence of the structural parameters of pristine commercial carbon blacks on their activity for oxygen reduction. Electrochim. Acta 2008, 53, 2925–2938. [Google Scholar] [CrossRef]
- Topkaya, R.; Kurtan, U.; Baykal, A.; Toprak, M.S. Polyvinylpyrrolidone (PVP)/MnFe2O4 nanocomposite: Sol-Gel autocombustion synthesis and its magnetic characterization. Ceram. Int. 2013, 39, 5651–5658. [Google Scholar] [CrossRef]
- McLeod, J.A.; Buling, A.; Green, R.J.; Boyko, T.D.; Skorikov, N.A.; Kurmaev, E.Z.; Neumann, M.; Finkelstein, L.D.; Ni, N.; Thaler, A.; et al. Effect of 3d doping on the electronic structure of BaFe2As2. J. Phys. Condens. Matter. 2012, 24, 215501. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Lee, S.J.; Biswas, P.; Lee, T.I.; Myoung, J.M. Solution-processed n-ZnO nanorod/p-Co3O4 nanoplate heterojunction light-emitting diode. Appl. Surf. Sci. 2017, 406, 192–198. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Lin, X.; Lee, J.; Li, S. Bipolar resistive switching in p-type Co3O4 nanosheets prepared by electrochemical deposition. Nanoscale Res. Lett. 2013, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Bingwa, N.; Bewana, S.; Ndolomingo, M.J.; Mawila, N.; Mogudi, B.; Ncube, P.; Carleschi, E.; Doyle, B.P.; Haumann, M.; Meijboom, R. Effect of alkali and alkaline earth metal dopants on catalytic activity of mesoporous cobalt oxide evaluated using a model reaction. Appl. Catal. A Gen. 2018, 555, 189–195. [Google Scholar] [CrossRef]
- Alders, D.; Sawatzky, G.; Voogt, F.; Hibma, T. Nonlocal screening effects in 2p X-ray photoemission spectroscopy of NiO (100). Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 7716–7719. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Döring, S.; Gorgoi, M.; Cramm, S.; Müller, M. Enhanced ferrimagnetism in auxetic NiFe2O4 in the crossover to the ultrathin-film limit. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 054418. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, B.; Jena, B.K.; Basu, S. Single Atom on the 2D Matrix: An Emerging Electrocatalyst for Energy Applications. ACS Omega 2020, 5, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.; Alves, J.P.C.; Lima, F.A.S.; Lira-Cantu, M.; Nogueira, A.F. Enhanced photovoltaic performance of inverted hybrid bulk-heterojunction solar cells using TiO 2/reduced graphene oxide films as electron transport layers. J. Photonics Energy 2015, 5, 057408. [Google Scholar] [CrossRef]
- West, W.; Carroll, B.H.; Whitcomb, D.H. The adsorption of sensitizing dyes in photographic emulsions. J. Phys. Chem. 1952, 56, 1054–1067. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 846, 3973–3993. [Google Scholar] [CrossRef]
- Mojović, Z.; Mudrinić, T.; Banković, P.; Jović-Jovičić, N.; Ivanović-Šašić, A.; Milutinović-Nikolić, A.; Jovanović, D. Oxygen reduction reaction on palladium-modified zeolite 13X. J. Solid State Electrochem. 2015, 19, 1993–2000. [Google Scholar] [CrossRef]
- He, X.; Yin, F.; Li, Y.; Wang, H.; Chen, J.; Wang, Y.; Chen, B. NiMnO3/NiMn2O4 Oxides Synthesized via the Aid of Pollen: Ilmenite/Spinel Hybrid Nanoparticles for Highly Efficient Bifunctional Oxygen Electrocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 26740–26757. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D. Nanostructured Oxygen Reduction Catalyst Designs to Reduce The Platinum Dependency of Polymer Electrolyte Fuel Cells. Ph.D. Thesis, University of Waterloo Library, Waterloo, ON, Canada, 24 July 2015. Available online: http://hdl.handle.net/10012/9483 (accessed on 30 August 2021).
- Chen, Z.; Zhao, B.; He, Y.C.; Wen, H.R.; Fu, X.Z.; Sun, R.; Wong, C.P. NiCo2O4 nanoframes with a nanosheet surface as efficient electrocatalysts for the oxygen evolution reaction. Mater. Chem. Front. 2018, 2, 1155–1164. [Google Scholar] [CrossRef]
- Hassan, D.; El-safty, S.; Khalil, K.A.; Dewidar, M.; El-magd, G.A. Carbon supported engineering NiCo2O4 hybrid nanofibers with enhanced electrocatalytic activity for oxygen reduction reaction. Matererials 2016, 9, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, A.B.; Abdel-Samad, H.S.; Rehim, S.S.A.; Hassan, H.H. Surface functionality and electrochemical investigations of a graphitic electrode as a candidate for alkaline energy conversion and storage devices. Sci. Rep. 2016, 6, 22056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Zhang, R.; Gao, R.; Shen, G.Q.; Pan, L.; Yao, Y.; Yu, K.; Zhang, X.; Zou, J.J. Metal-defected spinel MnxCo3-xO4 with octahedral Mn-enriched surface for highly efficient oxygen reduction reaction. Appl. Catal. B Environ. 2019, 244, 536–545. [Google Scholar] [CrossRef]
- Stamenković, V.; Schmidt, T.J.; Ross, P.N.; Marković, N.M. Surface segregation effects in electrocatalysis: Kinetics of oxygen reduction reaction on polycrystalline Pt3Ni alloy surfaces. J. Electroanal. Chem. 2003, 554–555, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Guo, S.; Zhao, Y.; Khan, I.U.; Zhang, X.; Li, K.; Lv, C. Single iron atoms anchored on activated carbon as active centres for highly efficient oxygen reduction reaction in air-cathode microbial fuel cell. J. Power Sources 2020, 450, 227683. [Google Scholar] [CrossRef]
- Yin, D.; Chen, Z.; Zhang, M. Sn-interspersed MoS2/C nanosheets with high capacity for Na+/K+ storage. J. Phys. Chem. Solids 2019, 126, 72–77. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, J.; Zhong, Q. Carbon-Supported Cu-Doped Mn-Co Spinel-Type Oxides Used as Cathodic Catalysts for the Oxygen Reduction Reaction in Dual-Chambered Microbial Fuel Cells. Energy Technol. 2015, 3, 48–54. [Google Scholar] [CrossRef]










| Material | Physicochemical Characteristics | Electrochemical Activity | |||||
|---|---|---|---|---|---|---|---|
| Crystal Size (nm) | Lattice Parameter (Å) | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) | Average Pore Size (nm) | Current Density (mAcm−2) | * Current Retention (%) | |
| x = 0 | 19.5 | 8.1084 | 63.19 | 0.1263 | 7.6 | −3.80 | 56.46 |
| x = 0.3 | 14.6 | 8.1357 | 55.17 | 0.1028 | 7.0 | −3.49 | 41.30 |
| x = 0.5 | 15.1 | 8.1480 | 83.42 | 0.2819 | 13.3 | −3.93 | 64.98 |
| x = 0.7 | 9.7 | 8.1402 | 44.49 | 0.2288 | 19.5 | −2.51 | 34.91 |
| x = 1 | 15.5 | 8.1281 | 36.63 | 0.2315 | 19.7 | −1.88 | 54.55 |
| Pt/C | - | - | - | 0.2668 | 4.85 | −4.42 | - |
| Mn0.5Ni0.5Co2O4/C | - | - | 209.52 | 0.1263 | 7.6 | −5.54 | 86.20 |
| Sample | O1 (eV) | O2 (eV) | O3 (eV) | FWHM (O1, eV) | FWHM (O2, eV) | FWHM (O3, eV) | O1 (%) | O2 (%) | O3 (%) |
| x = 0 | 529.49 | 531.14 | 533.24 | 1.23 | 2 | 2 | 39.1 | 51 | 9.9 |
| x = 0.3 | 529.62 | 531.27 | 533.27 | 1.29 | 2 | 2 | 40.4 | 42.8 | 16.8 |
| x = 0.5 | 529.8 | 531.36 | 533.42 | 1.29 | 2.1 | 2.1 | 44.3 | 41.6 | 14.1 |
| x = 0.7 | 530.06 | 531.56 | 533.59 | 1.41 | 1.8 | 1.8 | 70.2 | 25.6 | 4.2 |
| x = 1 | 530.2 | 531.8 | 533.4 | 1.35 | 1.57 | 1.9 | 71.4 | 17.6 | 11 |
| Mn0.5Ni0.5Co2O4/C | 529.79 | 531.23 | 533.36 | 1.25 | 2.2 | 2.2 | 41 | 45.6 | 13.4 |
| Sample Name | Solution Resistance (RC) (Ω) | Charge Transfer Resistance (RCT) (Ω) |
|---|---|---|
| NiCo2O4 | 6.621 | 230.3 |
| Mn0.3Ni0.7Co2O4 | 25.08 | 519.8 |
| Mn0.5Ni0.5Co2O4 | 2.776 | 272.43 |
| Mn0.7Ni0.3Co2O4 | 12.09 | 304.00 |
| MnCo2O4 | 105.2 | 799.9 |
| Mn0.5Ni0.5Co2O4/C | 1.076 | 92.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, T.; Dolla, T.H.; Gwebu, S.S.; Mashola, T.A.; Dlamini, L.T.; Carleschi, E.; Ndungu, P.; Maxakato, N.W. Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts 2021, 11, 1059. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091059
Matthews T, Dolla TH, Gwebu SS, Mashola TA, Dlamini LT, Carleschi E, Ndungu P, Maxakato NW. Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts. 2021; 11(9):1059. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091059
Chicago/Turabian StyleMatthews, Thabo, Tarekegn Heliso Dolla, Sandile Surprise Gwebu, Tebogo Abigail Mashola, Lihle Tshepiso Dlamini, Emanuela Carleschi, Patrick Ndungu, and Nobanathi Wendy Maxakato. 2021. "Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation" Catalysts 11, no. 9: 1059. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091059