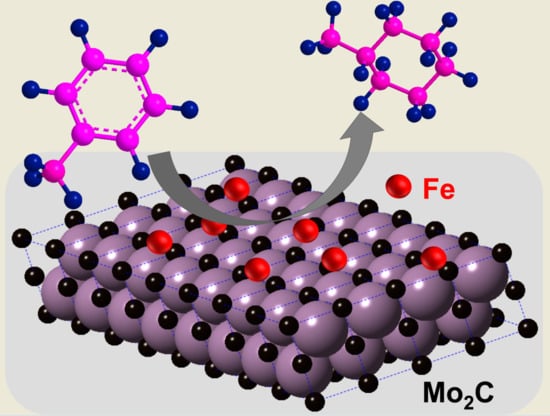
Low-Temperature Hydrogenation of Toluene Using an Iron-Promoted Molybdenum Carbide Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties and Morphology of the Fe/Mo2C Catalysts
2.2. Spatial Distribution of Fe-Promoter and Mo2C Phases in the Fe/Mo2C Catalysts
2.3. Fe-Mo2C Catalysts Performance
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Characterization
3.3. Catalyst Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Xue, N.; Liu, X. High-Strength Porous Millimeter Carbon Ball Having Controllable Internal Structure Useful in Fixed Bed Reactor for Catalytic Hydrogenation Reaction System. Patent CN112028050-A, 4 December 2020. [Google Scholar]
- Niu, M.; Tian, R.; Fan, Z.; Ji, P. Preparation of Gasoline and Diesel Oil by Mixing Coal Tar with Biomass Oil, Performing Catalytic Hydrogenation, Rectifying to Obtain Gasoline and Diesel Oil Product, and Using Coal Tar as Hydrogen Supply Solvent for Hydrogen Free Radical. Patent CN112029532-A, 4 December 2020. [Google Scholar]
- Cacciola, G.; Giordano, N.; Restuccia, G. Cyclohexane as a liquid phase carrier in hydrogen storage and transport. Int. J. Hydrog. Energy 1984, 9, 411–419. [Google Scholar] [CrossRef]
- Hamayun, M.H.; Maafa, I.M.; Hussain, M.; Aslam, R. Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production. Energies 2020, 13, 206. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Yuan, Y.; Chen, C.; Chaemchuen, S.; Verpoort, F. Palladium metallated shell layer of shell@core MOFs as an example of an efficient catalyst design strategy for effective olefin hydrogenation reaction. J. Catal. 2020, 392, 141–149. [Google Scholar] [CrossRef]
- Peyrovi, M.H.; Rostamikia, T.; Parsafard, N. Competitive Hydrogenation of Benzene in Reformate Gasoline over Ni Supported on SiO2, SiO2–Al2O3, and Al2O3 Catalysts: Influence of Support Nature. Energy Fuels 2018, 32, 11432–11439. [Google Scholar] [CrossRef]
- Weilhard, A.; Abarca, G.; Viscardi, J.; Prechtl, M.H.G.; Scholten, J.D.; Bernardi, F.; Baptista, D.L.; Dupont, J. Challenging Thermodynamics: Hydrogenation of Benzene to 1,3-Cyclohexadiene by Ru@Pt Nanoparticles. ChemCatChem 2017, 9, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Bi, H.; Tan, X.; Dou, R.; Pei, Y.; Qiao, M.; Sun, B.; Zong, B. Ru–B nanoparticles on metal–organic frameworks as excellent catalysts for hydrogenation of benzene to cyclohexane under mild reaction conditions. Green Chem. 2016, 18, 2216–2221. [Google Scholar] [CrossRef]
- Foppa, L.; Dupont, J. Benzene partial hydrogenation: Advances and perspectives. Chem. Soc. Rev. 2015, 44, 1886–1897. [Google Scholar] [CrossRef]
- Cai, J.; Bennici, S.; Shen, J.; Auroux, A. Influence of N addition in mesoporous carbons used as supports of Pt, Pd and Ru for toluene hydrogenation and iron oxide for benzene oxidation. React. Kinet. Mech. Catal. 2015, 115, 263–282. [Google Scholar] [CrossRef]
- Sun, H.; Guo, W.; Zhou, X.; Chen, Z.; Liu, Z.; Liu, S. Progress in Ru-Based Amorphous Alloy Catalysts for Selective Hydrogenation of Benzene to Cyclohexene. Chin. J. Catal. 2011, 32, 1–16. [Google Scholar] [CrossRef]
- Kazantsev, R.V.; Gaidai, N.A.; Nekrasov, N.V.; Tenchev, K.; Petrov, L.; Lapidus, A.L. Kinetics of benzene and toluene hydrogenation on a Pt/TiO2 catalyst. Kinet. Catal. 2003, 44, 529–535. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xu, Y.; Gao, X.; Dai, Y.; Tang, Y. Palladium-Incorporated α-MoC Mesoporous Composites for Enhanced Direct Hydrodeoxygenation of Anisole. Catalysts 2021, 11, 370. [Google Scholar] [CrossRef]
- Mamède, A.; Giraudon, J.-M.; Löfberg, A.; Leclercq, L. Hydrogenation of toluene over β-Mo2C in the presence of thiophene. Appl. Catal. A-Gen. 2002, 227, 73–82. [Google Scholar] [CrossRef]
- Da Costa, P.; Lemberton, J.-L.; Potvin, C.; Manoli, J.-M.; Perot, G.; Breysse, M.; Djega-Mariadassou, G. Tetralin hydrogenation catalyzed by Mo2C/Al2O3 and WC/Al2O3 in the presence of H2S. Catal. Today 2001, 65, 195–200. [Google Scholar] [CrossRef]
- Lin, S.D.; Song, C. Noble metal catalysts for low-temperature naphthalene hydrogenation in the presence of benzothiophene. Catal. Today 1996, 31, 93–104. [Google Scholar] [CrossRef]
- Stanislaus, A.; Cooper, B.H. Aromatic Hydrogenation Catalysis: A Review. Catal. Rev. 1994, 36, 75–123. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Alshammari, A.S.; Altamimi, R.M.; Kreyenschulte, C.; Pohl, M.M.; Lund, H.; Jagadeesh, R.V.; Beller, M. Cobalt-Nanoparticles Catalyzed Efficient and Selective Hydrogenation of Aromatic Hydrocarbons. ACS Catal. 2019, 9, 8581–8591. [Google Scholar] [CrossRef]
- Peyrovi, M.; Parsafard, N.; Mohammadian, Z. Benzene selective hydrogenation over supported Ni (nano-) particles catalysts: Catalytic and kinetics studies. Chin. J. Chem. Eng. 2018, 26, 521–528. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, J.; Song, C.; Li, G. HDS of dibenzothiophenes and hydrogenation of tetralin over a SiO2 supported Ni-Mo-S catalyst. Front. Chem. Sci. Eng. 2015, 9, 336–348. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Wu, K.; Zhang, K.; Jie, J.; Yang, Y. Preparation of Ni-Mo-S catalysts by hydrothermal method and their hydrodeoxygenation properties. Appl. Catal. A-Gen. 2015, 495, 8–16. [Google Scholar] [CrossRef]
- Schachtl, E.; Zhong, L.; Kondratieva, E.; Hein, J.; Gutiérrez, O.Y.; Jentys, A.; Lercher, J.A. Understanding Ni Promotion of MoS2/-Al2O3 and its Implications for the Hydrogenation of Phenanthrene. ChemCatChem 2015, 7, 4118–4130. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, K.; Li, L.; Wu, K.; Liu, P.; Yang, Y. Synthesis of Highly Active Co-Mo-S Unsupported Catalysts by a One-Step Hydrothermal Method for p-Cresol Hydrodeoxygenation. Ind. Eng. Chem. Res. 2014, 53, 19001–19009. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Singh, S.; Schachtl, E.; Kim, J.; Kondratieva, E.; Hein, J.; Lercher, J. Effects of the Support on the Performance and Promotion of (Ni)MoS2 Catalysts for Simultaneous Hydrodenitrogenation and Hydrodesulfurization. ACS Catal. 2014, 4, 1487–1499. [Google Scholar] [CrossRef]
- Choi, J.; Barnard, Z.G.; Zhang, S.; Hill, J.M. Ni catalysts supported on activated carbon from petcoke and their activity for toluene hydrogenation. Can. J. Chem. Eng. 2012, 90, 631–636. [Google Scholar] [CrossRef]
- Cho, A.; Moon, S.H. Development of Highly Active Co(Ni)Mo Catalysts for the Hydrodesulfurization of Dibenzothiophene Compounds. Catal. Surv. Asia 2010, 14, 64–74. [Google Scholar] [CrossRef]
- Moses, P.G.; Hinnemann, B.; Topsøe, H.; Nørskov, J.K. The effect of Co-promotion on MoS2 catalysts for hydrodesulfurization of thiophene: A density functional study. J. Catal. 2009, 268, 201–208. [Google Scholar] [CrossRef]
- Xiao, T.C.; York, A.P.; Megren, H.A.; Williams, C.V.; Wang, H.T.; Green, M.L. Preparation and characterisation of bimetallic cobalt and molybdenum carbides. J. Catal. 2001, 202, 100–109. [Google Scholar] [CrossRef]
- Dhandapani, B.; Clair, T.S.; Oyama, S. Simultaneous hydrodesulfurization, hydrodeoxygenation, and hydrogenation with molybdenum carbide. Appl. Catal. A-Gen. 1998, 168, 219–228. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.; Ye, Y.; Lin, L.; Wen, X.; Liu, P.; Chen, B.; et al. Atomic-layered Au clusters on alpha-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, X.; Zhang, Q.-H.; Tang, T.; Zhang, Y.; Gu, L.; Li, Y.; Bao, J.; Dai, Z.; Hu, J.-S. Engineering Mo/Mo2C/MoC hetero-interfaces for enhanced electrocatalytic nitrogen reduction. J. Mater. Chem. A 2020, 8, 8920–8926. [Google Scholar] [CrossRef]
- Kojima, R.; Aika, K.-I. Molybdenum nitride and carbide catalysts for ammonia synthesis. Appl. Catal. A-Gen. 2001, 219, 141–147. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.W.; Shi, C.; Ma, D. Highly Dispersed Copper over β-Mo2C as an Efficient and Stable Catalyst for the Reverse Water Gas Shift (RWGS) Reaction. ACS Catal. 2016, 7, 912–918. [Google Scholar] [CrossRef]
- Xu, W.; Ramirez, P.J.; Stacchiola, D.; Rodriguez, J.A. Synthesis of α-MoC1-x and β-MoCy Catalysts for CO2 Hydrogenation by Thermal Carburization of Mo-oxide in Hydrocarbon and Hydrogen Mixtures. Catal. Lett. 2014, 144, 1418–1424. [Google Scholar] [CrossRef]
- Dongil, A.B.; Zhang, Q.; Pastor-Pérez, L.; Ramírez-Reina, T.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of Cu and Cs in the β-Mo2C System for CO2 Hydrogenation to Methanol. Catalysts 2020, 10, 1213. [Google Scholar] [CrossRef]
- Shilov, I.N.; Smirnov, A.A.; Bulavchenko, O.A.; Yakovlev, V.A. Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation. Catalysts 2018, 8, 560. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.A.; Liu, P.; Takahashi, Y.; Nakamura, K.; Viñes, F.; Illas, F. Desulfurization Reactions on Surfaces of Metal Carbides: Photoemission and Density–Functional Studies. Top. Catal. 2010, 53, 393–402. [Google Scholar] [CrossRef]
- Piskorz, W.; Adamski, G.; Kotarba, A.; Sojka, Z.; Sayag, C.; Djéga-Mariadassou, G. Hydrodenitrogenation of indole over Mo2C catalyst: Insights into mechanistic events through DFT modeling. Catal. Today 2007, 119, 39–43. [Google Scholar] [CrossRef]
- Diaz, B.; Sawhill, S.J.; Bale, D.H.; Main, R.; Phillips, D.C.; Korlann, S.; Self, R.; Bussell, M.E. Hydrodesulfurization over supported monometallic, bimetallic and promoted carbide and nitride catalysts. Catal. Today 2003, 86, 191–209. [Google Scholar] [CrossRef]
- Lewandowski, M.; Janus, R.; Wądrzyk, M.; Szymańska-Kolasa, A.; Sayag, C.; Djéga-Mariadassou, G. On Catalytic Behavior of Bulk Mo2C in the Hydrodenitrogenation of Indole over a Wide Range of Conversion Thereof. Catalysts 2020, 10, 1355. [Google Scholar] [CrossRef]
- Deng, Y.; Ge, Y.; Xu, M.; Yu, Q.; Xiao, D.; Yao, S.; Ma, D. Molybdenum Carbide: Controlling the Geometric and Electronic Structure of Noble Metals for the Activation of O–H and C–H Bonds. Acc. Chem. Res. 2019, 52, 3372–3383. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically Dispersed Ni/α-MoC Catalyst for Hydrogen Production from Methanol/Water. J. Am. Chem. Soc. 2020, 143, 309–317. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.-W.; et al. Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Raghav, H.; Konathala, L.S.K.; Mishra, N.; Joshi, B.; Goyal, R.; Agrawal, A.; Sarkar, B. Fe-decorated hierarchical molybdenum carbide for direct conversion of CO2 into ethylene: Tailoring activity and stability. J. CO2 Util. 2021, 50, 101607. [Google Scholar] [CrossRef]
- Koverga, A.A.; Gómez-Marín, A.M.; Dorkis, L.; Florez, E.; Ticianelli, E.A. Role of Transition Metals on TM/Mo2C Composites: Hydrogen Evolution Activity in Mildly Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2020, 12, 27150–27165. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zou, J. CO Hydrogenation over Transition Metals (Fe, Co, or Ni) Modified K/Mo2C Catalysts. J. Catal. 2013, 2013, 195920. [Google Scholar] [CrossRef] [Green Version]
- Suppino, R.S.; Landers, R.; Cobo, A.J.G. Effects of the activation method on the performance of base metal catalysts prepared by wet impregnation for toluene hydrogenation in liquid phase. React. Kinet. Mech. Catal. 2015, 114, 295–309. [Google Scholar] [CrossRef]
- Yoon, K.J.; Vannice, M.A. Benzene hydrogenation over iron: II. Reaction model over unsupported and supported catalysts. J. Catal. 1983, 82, 457–468. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Kusakabe, K.; Abudula, A. Highly-efficient steam reforming of methanol over copper modified molybdenum carbide. RSC Adv. 2014, 4, 44175–44184. [Google Scholar] [CrossRef]
- Alayoglu, S.; Somorjai, G.A. Ambient Pressure X-ray Photoelectron Spectroscopy for Probing Monometallic, Bimetallic and Oxide-Metal Catalysts Under Reactive Atmospheres and Catalytic Reaction Conditions. Top. Catal. 2016, 59, 420–438. [Google Scholar] [CrossRef]
- Tao, F.; Grass, M.E.; Zhang, Y.; Butcher, D.R.; Renzas, J.R.; Liu, Z.; Chung, J.Y.; Mun, B.S.; Salmeron, M.; Somorjai, G.A. Reaction-Driven Restructuring of Rh-Pd and Pt-Pd Core-Shell Nanoparticles. Science 2008, 322, 932–934. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.J.; Jablonski, A.; Salvat, F. NIST databases with electron elastic-scattering cross sections, inelastic mean free paths, and effective attenuation lengths. Surf. Interface Anal. 2005, 37, 1068–1071. [Google Scholar] [CrossRef]
- Idczak, K.; Idczak, R. Investigation of Surface Segregation in Fe-Cr-Si Alloys by XPS. Met. Mater. Trans. A 2020, 51, 3076–3089. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Lin, L.; Liao, W.; Rui, N.; Li, N.; Liu, Z.; Cen, J.; Zhang, F.; Li, X.; Song, L.; et al. Exploring Metal–Support Interactions To Immobilize Subnanometer Co Clusters on γ–Mo2N: A Highly Selective and Stable Catalyst for CO2 Activation. ACS Catal. 2019, 9, 9087–9097. [Google Scholar] [CrossRef]
- Frauwallner, M.-L.; López-Linares, F.; Lara-Romero, J.; Scott, C.E.; Ali, V.; Hernández, E.; Pereira-Almao, P. Toluene hydrogenation at low temperature using a molybdenum carbide catalyst. Appl. Catal. A-Gen. 2011, 394, 62–70. [Google Scholar] [CrossRef]
- Lee, J.S.; Yeom, M.H.; Park, K.Y.; Nam, I.-S.; Chung, J.S.; Kim, Y.G.; Moon, S.H. Preparation and benzene hydrogenation activity of supported molybdenum carbide catalysts. J. Catal. 1991, 128, 126–136. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Deng, Y.; Xu, M.; Artiglia, L.; Wen, W.; Gao, R.; Chen, B.; Yao, S.; Zhang, X.; et al. A stable low-temperature H2-production catalyst by crowding Pt on α-MoC. Nature 2021, 589, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Mudiyanselage, K.; Senanayake, S.; Feria, L.; Kundu, S.; Baber, A.E.; Graciani, J.; Vidal, A.B.; Agnoli, S.; Evans, J.; Chang, R.; et al. Importance of the Metal-Oxide Interface in Catalysis: In Situ Studies of the Water-Gas Shift Reaction by Ambient-Pressure X-ray Photoelectron Spectroscopy. Angew. Chem. Int. Ed. 2013, 52, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bartholomew, C.H. Temperature-Programmed Hydrogenation (TPH) and in Situ Mössbauer Spectroscopy Studies of Carbonaceous Species on Silica-Supported Iron Fischer−Tropsch Catalysts. J. Phys. Chem. B 2005, 109, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Dong, Q.; Han, Y.; Mao, B.-H.; Zhang, H.; Karlsson, P.G.; Åhlund, J.; Tai, R.-Z.; Yu, Y.; Liu, Z. An APXPS endstation for gas–solid and liquid–solid interface studies at SSRF. Nucl. Sci. Tech. 2019, 30, 81. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Liu, X.; Xu, J.; Zhang, H.; Liu, X.; Li, P.; Wen, X.; Yang, Y.; Li, Y. Low-Temperature Hydrogenation of Toluene Using an Iron-Promoted Molybdenum Carbide Catalyst. Catalysts 2021, 11, 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091079
Zhou S, Liu X, Xu J, Zhang H, Liu X, Li P, Wen X, Yang Y, Li Y. Low-Temperature Hydrogenation of Toluene Using an Iron-Promoted Molybdenum Carbide Catalyst. Catalysts. 2021; 11(9):1079. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091079
Chicago/Turabian StyleZhou, Song, Xi Liu, Jian Xu, Hui Zhang, Xiaosong Liu, Pengcheng Li, Xiaodong Wen, Yong Yang, and Yongwang Li. 2021. "Low-Temperature Hydrogenation of Toluene Using an Iron-Promoted Molybdenum Carbide Catalyst" Catalysts 11, no. 9: 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091079