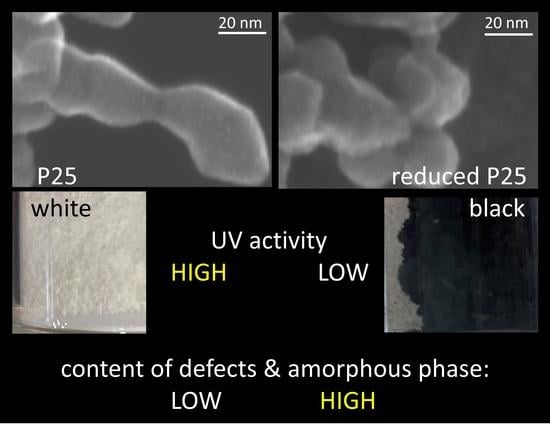
Is Black Titania a Promising Photocatalyst?
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Selected Titania Materials
3.2. Preparation of Black Titania Particles
3.3. Characterization of Black Titania Particles
3.4. Photocatalytic Activity Tests
3.4.1. Methanol Dehydrogenation Test
3.4.2. Acetic Acid Decomposition Test
3.4.3. Phenol Oxidation Test
3.5. Bactericidal Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Influence of the preparation methods of titanium dioxide on the photocatalytic degradation of phenol in aqueous dispersion. J. Phys. Chem. 1990, 94, 829–832. [Google Scholar] [CrossRef]
- Morawski, A.; Grzechulska, J.; Kałucki, K. A new method for preparation of potassium-pillared layered titanate applied in photocatalysis. J. Phys. Chem. Solids 1996, 57, 1011–1017. [Google Scholar] [CrossRef]
- Pichat, P. A Brief Survey of the Potential Health Risks of TiO2 Particles and TiO2- Containing Photocatalytic or Non-Photocatalytic Materials. J. Adv. Oxid. Technol. 2010, 13, 238–246. [Google Scholar] [CrossRef]
- Bahnemann, D.; Henglein, A.; Lilie, J.; Spanhel, L. Flash photolysis observation of the absorption spectra of trapped positive holes and electrons in colloidal titanium dioxide. J. Phys. Chem. 1984, 88, 709–711. [Google Scholar] [CrossRef]
- Kolen’Ko, Y.; Churagulov, B.; Kunst, M.; Mazerolles, L.; Colbeau-Justin, C. Photocatalytic properties of titania powders prepared by hydrothermal method. Appl. Catal. B Environ. 2004, 54, 51–58. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Sclafani, A.; Schiavello, M. Activity of chromium-ion-doped titania for the dinitrogen photoreduction to ammonia and for the phenol photodegradation. J. Phys. Chem. 1988, 92, 6710–6713. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Rec. Patent. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Kouamé, N.A.; Alaoui, O.T.; Herissan, A.; Larios, E.; José-Yacaman, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Visible light-induced photocatalytic activity of modified titanium(iv) oxide with zero-valent bismuth clusters. New J. Chem. 2015, 39, 2316–2322. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.-H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, D.; Kisch, H. The Nature of Nitrogen-Modified Titanium Dioxide Photocatalysts Active in Visible Light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef] [PubMed]
- Beranek, R.; Kisch, H. A Hybrid Semiconductor Electrode for Wavelength-Controlled Switching of the Photocurrent Direction. Angew. Chem. Int. Ed. 2008, 47, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Kowalska, E.; Juodkazis, S.; Henkiel, P.; Kowalski, D. Site-Selective Au+ Electroreduction in Titania Nanotubes for Electrochemical and Plasmonic Applications. ACS Appl. Nano Mater. 2022, 5, 7696–7703. [Google Scholar] [CrossRef]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef] [Green Version]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on titanium dioxide powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor-Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Kamat, P.V. Improving the Photoelectrochemical Performance of Nanostructured TiO2 Films by Adsorption of Gold Nanoparticles. J. Phys. Chem. B 2000, 104, 10851–10857. [Google Scholar] [CrossRef]
- Sakai, N.; Sakai, T.; Matsubara, K.; Tatsuma, T. Layer-by-layer assembly of gold nanoparticles with titania nanosheets: Control of plasmon resonance and photovoltaic properties. J. Mater. Chem. 2010, 20, 4371–4378. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Takahashi, Y.; Tatsuma, T. Plasmon-Resonance-Based Generation of Cathodic Photocurrent at Electrodeposited Gold Nanoparticles Coated with TiO2 Films. ChemPhysChem 2009, 10, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Kominami, H.; Tanaka, A.; Hashimoto, K. Gold nanoparticles supported on cerium(IV) oxide powder for mineralization of organic acids in aqueous suspensions under irradiation of visible light of λ = 530 nm. Appl. Catal. A Gen. 2011, 397, 121–126. [Google Scholar] [CrossRef]
- Benz, D.; Felter, K.M.; Koeser, J.; Thöming, J.; Mul, G.; Grozema, F.C.; Hintzen, H.T.; Kreutzer, M.T.; van Ommen, J.R. Assessing the Role of Pt Clusters on TiO2 (P25) on the Photocatalytic Degradation of Acid Blue 9 and Rhodamine B. J. Phys. Chem. C 2020, 124, 8269–8278. [Google Scholar] [CrossRef] [Green Version]
- Kmetykó, A.; Mogyorosi, K.; Pusztai, P.; Radu, T.; Konya, Z.; Dombi, A.; Hernadi, K. Photocatalytic H2 Evolution Using Different Commercial TiO2 Catalysts Deposited with Finely Size-Tailored Au Nanoparticles: Critical Dependence on Au Particle Size. Materials 2014, 7, 7615–7633. [Google Scholar] [CrossRef] [Green Version]
- Tóth, Z.-R.; Kovács, G.; Hernádi, K.; Baia, L.; Pap, Z. The investigation of the photocatalytic efficiency of spherical gold nanocages/TiO2 and silver nanospheres/TiO2 composites. Sep. Purif. Technol. 2017, 183, 216–225. [Google Scholar] [CrossRef]
- Dörr, T.S.; Deilmann, L.; Haselmann, G.; Cherevan, A.; Zhang, P.; Blaha, P.; de Oliveira, P.W.; Kraus, T.; Eder, D. Ordered Mesoporous TiO2 Gyroids: Effects of Pore Architecture and Nb-Doping on Photocatalytic Hydrogen Evolution under UV and Visible Irradiation. Adv. Energy Mater. 2018, 8, 1802566. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, N.P.; Kafizas, A.; Quesada-Cabrera, R.; Sotelo-Vazquez, C.; Bawaked, S.M.; Mokhtar, M.; Al Thabaiti, S.A.; Obaid, A.Y.; Basahel, S.N.; Durrant, J.R.; et al. Ultraviolet Radiation Induced Dopant Loss in a TiO2 Photocatalyst. ACS Catal. 2017, 7, 1485–1490. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D.; Pask, J.A. Kinetics of the Anatase-Rutile Transformation. J. Am. Ceram. Soc. 1965, 48, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Gennari, F.C.; Pasquevich, D.M. Kinetics of the anatase–rutile transformation in TiO2 in the presence of Fe2O3. J. Mater. Sci. 1998, 33, 1571–1578. [Google Scholar] [CrossRef]
- Plugaru, R.; Cremades, A.; Piqueras, J. The effect of annealing in different atmospheres on the luminescence of polycrystalline TiO2. J. Phys. Condens. Matter 2004, 16, S261–S268. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in Black Titania: A New Material for Advanced Photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Rajaraman, T.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Mansingh, S.; Babu, P.; Parida, K. Black titania an emerging photocatalyst: Review highlighting the synthesis techniques and photocatalytic activity for hydrogen generation. Nanoscale Adv. 2021, 3, 5487–5524. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Kowalska, E. Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts 2021, 11, 978. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Correction: Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G. Laser irradiation in water for the novel, scalable synthesis of black TiOx photocatalyst for environmental remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicka, E.; Zhou, X.; Denisov, N.; Yoo, Y.E.; Fehn, D.; Liu, N.; Meyer, K.; Schmuki, P. Self-enhancing H2 evolution from TiO2 nanostructures under illumination. ChemSusChem 2019, 12, 1900–1905. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Morales-Torres, S. Synthesis of TixOy nanocrystals in mild synthesis conditions for the degradation of pollutants under solar light. Appl. Catal. B Environ. 2019, 241, 385–392. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.-L.; Pan, J.-H.; Li, B.-R.; Wu, L.-G.; Jiang, B.-Q. Hydrothermal reduction of commercial P25 photocatalysts to expand their visible-light response and enhance their performance for photodegrading phenol in high-salinity wastewater. Appl. Surf. Sci. 2019, 480, 896–904. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Yang, F.; Wang, Z.; Xu, Y.; Tian, G.; Pan, K.; Jiang, B.; Zhou, W. Homojunction and defect synergy-mediated electron–hole separation for solar-driven mesoporous rutile/anatase TiO2 microsphere photocatalysts. RSC Adv. 2019, 9, 7870–7877. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ahmed, R.; Klein, D.; Cap, S.; Freedy, K.; McDonnell, S.; Zangari, G. Improving photo-oxidation activity of water by introducing Ti3+ in self-ordered TiO2 nanotube arrays treated with Ar/NH3. J. Power Sources 2019, 414, 242–249. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.-C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.-A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, G.; Lin, T.; Hong, Z.; Wang, J.; Huang, F. Molten salt assisted synthesis of black titania hexagonal nanosheets with tuneable phase composition and morphology. RSC Adv. 2015, 5, 85928–85932. [Google Scholar] [CrossRef]
- Leshuk, T.; Parviz, R.; Everett, P.; Krishnakumar, H.; Varin, R.A. Photocatalytic activity of hydrogenated TiO2. ACS Appl. Mater. Interfaces 2013, 5, 1892–1895. [Google Scholar] [CrossRef]
- Ren, W.; Yan, Y.; Zeng, L.; Shi, Z.; Gong, A.; Schaaf, P.; Wang, D.; Zhao, J.; Zou, B.; Yu, H.; et al. A Near Infrared Light Triggered Hydrogenated Black TiO2 for Cancer Photothermal Therapy. Adv. Healthc. Mater. 2015, 4, 1526–1536. [Google Scholar] [CrossRef]
- Konig, K. Multiphoton microscopy in life sciences. J. Microsc. 2000, 200, 83–104. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, S.; Paunesku, T.; Gleber, S.C.; Liu, W.C.; Doty, C.B.; Mak, R.; Deng, J.; Jin, Q.; Lai, B.; et al. Epidermal growth factor receptor targeted nuclear delivery and high-resolution whole cell x-ray imaging of Fe3O4@TiO2 nanoparticles in cancer cells. ACS Nano 2013, 7, 10502–10517. [Google Scholar] [CrossRef]
- Rozhkova, E.A.; Ulasov, I.; Lai, B.; Dimitrijevic, N.M.; Lesniak, M.S.; Rajh, T. A High-Performance Nanobio Photocatalyst for Targeted Brain Cancer Therapy. Nano Lett. 2009, 9, 3337–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.; Lau, J.W.; Do, T.C.; Zhang, Z.; Xing, B. Near-Infrared Light Brightens Bacterial Disinfection: Recent Progress and Perspectives. ACS Appl. Bio Mater. 2021, 4, 3937–3961. [Google Scholar] [CrossRef]
- Urgnani, J.; Torres, F.J.; Palumbo, M.; Baricco, M. Hydrogen release from solid state NaBH4. Int. J. Hydrogen Energy 2008, 33, 3111–3115. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. Oxygen-Deficient Black Titania for Synergistic/Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano 2018, 12, 4545–4555. [Google Scholar] [CrossRef]
- Li, T.; Shen, Z.; Shu, Y.; Li, X.; Jiang, C.; Chen, W. Facet-dependent evolution of surface defects in anatase TiO2 by thermal treatment: Implications for environmental applications of photocatalysis. Environ. Sci. Nano 2019, 6, 1740–1753. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A.; MacKenzie, K.J.D. Effect of Silica Additive on the Anatase-to-Rutile Phase Transition. J. Am. Ceram. Soc. 2001, 84, 1591–1596. [Google Scholar] [CrossRef]
- Albetran, H.; Vega, V.; Prida, V.M.; Low, I.-M. Dynamic Diffraction Studies on the Crystallization, Phase Transformation, and Activation Energies in Anodized Titania Nanotubes. Nanomaterials 2018, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Ghuman, K.K. Mechanistic insights into water adsorption and dissociation on amorphous TiO2-based catalysts. Sci. Technol. Adv. Mater. 2018, 19, 44–52. [Google Scholar] [CrossRef]
- Zhu, G.; Shan, Y.; Lin, T.; Zhao, W.; Xu, J.; Tian, Z.; Zhang, H.; Zheng, C.; Huang, F. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Sugiyama, N.; Murakami, S.-Y.; Kominami, H.; Kera, Y.; Noguchi, H.; Uosaki, K.; Torimoto, T.; Ohtani, B. Quantitative analysis of defective sites in titanium(IV) oxide photocatalyst powders. Phys. Chem. Chem. Phys. 2003, 5, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Park, J.H. Surface Localization of Defects in Black TiO2: Enhancing Photoactivity or Reactivity. J. Phys. Chem. Lett. 2017, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Mahjouri-Samani, M.; Eres, G.; Sachan, R.; Yoon, M.; Chisholm, M.F.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; et al. Structure and Formation Mechanism of Black TiO2 Nanoparticles. ACS Nano 2015, 9, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef]
- Min, B.K.; Wallace, W.T.; Goodman, D.W. Support effects on the nucleation, growth, and morphology of gold nano-clusters. Surf. Sci. 2006, 600, L7–L11. [Google Scholar] [CrossRef]
- Bielan, Z.; Dudziak, S.; Sulowska, A.; Pelczarski, D.; Ryl, J.; Zielińska-Jurek, A. Preparation and Characterization of Defective TiO2. The Effect of the Reaction Environment on Titanium Vacancies Formation. Materials 2020, 13, 2763. [Google Scholar] [CrossRef]
- Zywitzki, D.; Jing, H.; Tüysüz, H.; Chan, C.K. Correction: High surface area, amorphous titania with reactive Ti3+ through a photo-assisted synthesis method for photocatalytic H2 generation. J. Mater. Chem. A 2017, 5, 10957–10967. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, Y.; Li, J.; Hu, Z.; Zhao, H.; Xie, W.; Wei, Z. Synthesis of black TiO2 with efficient visible-light photocatalytic activity by ultraviolet light irradiation and low temperature annealing. Mater. Res. Bull. 2018, 98, 280–287. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the Relative Concentration Ratio of Bulk Defects to Surface Defects in TiO2 Nanocrystals Leads to High Photocatalytic Efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, X.; Nguyen, N.T.; Peters, K.; Zoller, F.; Hwang, I.; Schneider, C.; Miehlich, M.E.; Freitag, D.; Meyer, K.; et al. Black Magic in Gray Titania: Noble-Metal-Free Photocatalytic H2 Evolution from Hydrogenated Anatase. ChemSusChem 2017, 10, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.A.; Kozlov, D.A.; Kolesnik, I.V.; Poluboyarinov, A.S.; Becerikli, A.E.; Grünert, W.; Garshev, A.V. The amorphous phase in titania and its influence on photocatalytic properties. Appl. Catal. B Environ. 2016, 195, 39–47. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Roszek, K. Underestimated Properties of Nanosized Amorphous Titanium Dioxide. Int. J. Mol. Sci. 2022, 23, 2460. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G. Bacterial Inhibition by Electrical Stimulation. Adv. Wound Care 2014, 3, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication–hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [Green Version]
- Nitta, A.; Takase, M.; Takashima, M.; Murakami, N.; Ohtani, B. A fingerprint of metal-oxide powders: Energy-resolved distribution of electron traps. Chem. Commun. 2016, 52, 12096–12099. [Google Scholar] [CrossRef]












| Sample | Composition (%) | O/Ti Ratio | Titanium Form (%) | Oxygen Form (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Ti | O | Ti3+ | Ti4+ | O–H | M–OH/C=O | TiO2 | ||
| P25 | 27.6 | 72.4 | 2.6 | 3.6 | 96.4 | 8.6 | 34.6 | 56.8 |
| P25-R1 | 16.3 | 83.7 | 5.1 | 6.1 | 93.9 | 30.3 | 38.7 | 30.9 |
| P25-R2 | 20.0 | 80.0 | 4.0 | 3.5 | 96.5 | 23.4 | 34.7 | 41.9 |
| OAP | 14.3 | 85.7 | 6.0 | 2.6 | 97.4 | 28.4 | 42.0 | 29.6 |
| OAP-R1 | 30.5 | 69.5 | 2.3 | 4.4 | 95.6 | 1.0 | 30.9 | 68.1 |
| DAP | 13.7 | 86.3 | 6.3 | 4.8 | 95.2 | 39.8 | 40.2 | 30.1 |
| DAP-R1 | 21.9 | 78.1 | 3.6 | 4.8 | 95.2 | 29.4 | 30.1 | 41.9 |
| Sample Name | Pristine | Modified (“Black”) | ||
|---|---|---|---|---|
| R1 | R2 | R3 | ||
| P25 | 73 | 211 | 97 | 87 |
| TIO-2 | 29 | 173 | 135 | - |
| ST01 | 84 | 120 | 103 | - |
| OAP | 52 | 184 | - | - |
| DAP | 20 | 115 | - | - |
| Sample Name | Crystalline Composition (%) | Crystallite Size (nm) | |||
|---|---|---|---|---|---|
| Anatase | Rutile | NC | Anatase | Rutile | |
| P25 | 77.2 | 13.2 | 9.6 | 25 | 39.6 |
| P25-R3 | 63.2 | 11.3 | 25.5 | 20.3 | 23.3 |
| TIO-2 | 83.1 | 0 | 16.9 | 68.9 | - |
| TIO-2-R2 | 29.5 | 0 | 70.5 | 13.5 | - |
| ST01 | 72.8 | 0 | 27.2 | 7.6 | - |
| ST01-R1 | 66.1 | 0 | 33.9 | 4.6 | - |
| OAP | 78.0 | 0 | 22.0 | 27.0 | - |
| OAP-R1 | 61.8 | 0 | 38.2 | 24.1 | - |
| DAP | 90.8 | 0.0 | 6.2 | 67.0 | - |
| DAP-R1 | 55.5 | 0 | 44.5 | 63.8 | - |
| Sample Name | Composition (%) | Crystallite Size * (nm) | BET | ETs ** | ||
|---|---|---|---|---|---|---|
| A | R | NC | (m2 g−1) | (μmol g−1) | ||
| P25 | 76–80 | 13–15 | 6–11 | 25 | 59 | 114 |
| TIO-2 | 91 | 0 | 17 | 68.9 | 17 | 32 |
| ST01 | 80 | 0 | 27 | 8 | 344 | 94 |
| OAP | 78 | 0 | 22 | 27 | 126 | 34 |
| DAP | 91 | 3 | 6 | 67 | 16 | 19 |
| Sample Name | Mass Ratio of TiO2/NaBH4 | Calcination Temperature (K) | Calcination Time (min) |
|---|---|---|---|
| P25-R1 | 5:1 | 598 | 60 |
| P25-R2 | 10:1 | 523 | 60 |
| P25-R3 | 10:1 | 523 | 5 |
| TIO-2-R1 | 3.333:1 | 598 | 60 |
| TIO-2-R2 | 5:1 | 598 | 60 |
| ST01-R1 | 5:1 | 598 | 60 |
| ST01-R2 | 5:1 | 523 | 60 |
| DAP-R1 | 5:1 | 523 | 30 |
| OAP-R1 | 5:1 | 598 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Akanda, M.M.A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Is Black Titania a Promising Photocatalyst? Catalysts 2022, 12, 1320. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12111320
Janczarek M, Endo-Kimura M, Wang K, Wei Z, Akanda MMA, Markowska-Szczupak A, Ohtani B, Kowalska E. Is Black Titania a Promising Photocatalyst? Catalysts. 2022; 12(11):1320. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12111320
Chicago/Turabian StyleJanczarek, Marcin, Maya Endo-Kimura, Kunlei Wang, Zhishun Wei, Md Mahbub A. Akanda, Agata Markowska-Szczupak, Bunsho Ohtani, and Ewa Kowalska. 2022. "Is Black Titania a Promising Photocatalyst?" Catalysts 12, no. 11: 1320. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12111320