Pervaporation Membrane-Catalytic Reactors for Isoamyl Acetate Production
Abstract
:1. Introduction
2. Thermodynamics, Reaction Kinetics, and Membrane Transport Models for PVMR Simulation
2.1. Thermodynamic Model
2.2. Reaction Kinetics
2.3. Membrane Transport Model
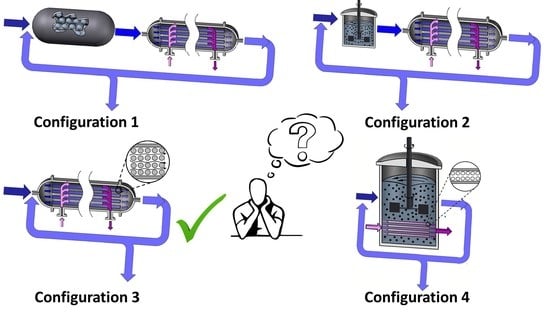
3. Pervaporation Membrane Reactor Models and Anticipated Unit Configurations
- i.
- Steady state operation.
- ii.
- Constant density (liquid phase reaction).
- iii.
- For all four PVMR configurations, the membrane is entirely unreactive (i.e., the reaction ends once the liquid mixture leaves the reaction unit). Transport resistance in the membrane support structure in the permeate side, and concentration polarization effects in the tube-side are considered negligible.
- iv.
- The chemical species evolving in the reactor side in the FB, in both the FBR+PVMU (Configuration 1) and the IFBPVMR (Configuration 3) can be modeled using an ideal plug flow reactor model. This is acceptable given the proper selection of catalyst particle size and fluid dynamic conditions, making concentration changes, a function of the length of the reactor only.
- v.
- The chemical species in the SR, in both the SR+PVMU (Configuration 2) and the ISPVMR (Configuration 4) unit can be simulated by using an ideal perfectly stirred tank reactor, with a uniformly suspended catalyst. This is suitable given the efficient stirring that guarantees that the catalyst density, composition, temperature, and reaction rate are constant throughout the reactor volume.
- vi.
- For all four configurations, the chemical species transport in the pervaporator unit (permeate side) can be assumed to evolve with a plug flow pattern. This is adequate given the carefully selected inert particle sizes and fluid-dynamic conditions that allow species concentration changes to occur, in the unit axial position only. In the particular case of Configurations 1 and 2, this same assumption also applies to the retentate side of the pervaporator, given the loaded inert particles and fluid dynamics.
3.1. Modeling the Fixed Bed Reactor and Pervaporation Membrane Unit (FBR+PVMU) or Configuration 1
3.2. Modeling the Slurry Reactor and the Pervaporation Membrane Unit (SR+PVMU) or Configuration 2
3.3. Modeling an Integrated Fixed Bed and Pervaporation Membrane Reactor (IFBPVMR) or Configuration 3
3.4. Modeling an Integrated Slurry and Pervaporation Membrane Reactor (ISPVMR) or Configuration 4
4. Dimensionless Numbers and Design Variables
5. Results and Discussion
5.1. FBR+PVMU (Configuration 1)
5.2. SR+PVMU (Configuration 2)
5.3. IFBPVMR (Configuration 3)
5.4. ISPVMR (Configuration 4)
5.5. Summary of Design Criteria
- The membrane reactor design should have, as a main objective, to enhance ester yield, with less consideration given to the conversion of the limiting reagent.
- The design of a membrane reactor for liquid phase esterification can only be considered suitable if the ester yield surpasses chemical equilibrium values, achieved in conventional reactors (e.g., unit without membrane).
- The specific design selected must ensure that a retentate water concentration is low enough not to require further ester purification by azeotropic distillation.
- The simulations of the different catalytic reactor-pervaporator membrane configurations show that the one which performs best is the integrated IFBPVMR (Configuration 3), which demonstrates that: (a) for a set Da, there is a best Ω*, where the ester yield becomes a maximum, (b) for a set Ω, there is an optimum Da*, at which the ester yield reaches a maximum level, (c) for a set Ω, there is a Da* where the retentate water mole fraction reaches a highest value.
- The simulation of the best performing integrated IFBPVMRs further confirms that for set Ω and Da values in a reactor with recycle, there is an R* at which the ester yields reach maximum levels.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Membrane area (m2) |
| Am | Total membrane area (m2) |
| a | NRTL adjustable parameter (-) in Equation (2). Thermodynamic activity (-) in Equations (19)–(23). |
| B | correction factor for the permeant activity inside the membrane (-) |
| b | NRTL adjustable parameter (K) |
| c | NRTL adjustable parameter (-) |
| ci | Retentate concentration of the i species (mol/m3) |
| D | Diffusion coefficient (m2/h) |
| D′ | Apparent molar diffusion coefficient in the membrane (m/h) |
| Dim | Diffusivity of i in the membrane (m2/h) |
| Da | Damköhler number (-) |
| d | NRTL adjustable parameter (K−1) |
| e | NRTL adjustable parameter (-) |
| F | Molar flow (mol/h) |
| f | NRTL adjustable parameter (K−1) |
| G | NRTL Excess Gibbs Energy (J/mol) |
| ji | Molar flux of I (mol/(m2.h)) |
| Keq | Equilibrium constant (-) |
| k1,hom | Kinetic constant for homogeneous reaction (mol/L.h) |
| k1,cat | Kinetic constant for catalytic reaction (mol/gcat.h) |
| N | Flux of permeation trough membrane (mol/m2.h) |
| P | Pressure (bar) |
| Overall reaction rate (-) | |
| R | Universal gas constant (J/mol*K) in Equations (15) and (18)–(20). Recycle ratio (-) fraction of the molar flow of i species exiting the reactor-membrane-unit, (mol/h), recycled back to reactor entrance, 0 < R < 1). |
| RE | Ester yield (-) |
| r | Reaction rate (mol/Lh for homogeneous or mol/gh for heterogeneous reaction) |
| s | +1 for countercurrent flow and −1 for parallel flow |
| T | Temperature (K) |
| V | Reactor volume (L) |
| Vcat | Volume of catalyst used (m3) |
| VR | Total reactor volume (L) |
| Wcat | weight of catalyst used (kg) |
| X | Overall reactant conversion (-) |
| x | Liquid phase molar fraction (mol/mol) |
| Y | Dimensionless molar flow/Molar flow of “i” species/Total molar flow (-) |
| z | Membrane thickness (m) |
| Greek symbol | |
| α | NRTL Non-randomness parameter (-) |
| αm | membrane area/reactor volume ratio (m2/L) |
| γ | Activity coefficient (-) |
| ε | Packed bed porosity (L/L) |
| θ | Acetic acid/Isoamyl alcohol molar feed ratio (-) |
| μ | Chemical potential (J/mol) |
| ν | Stoichiometric coefficient (mol/mol) |
| ξ | Dimensionless reactor volume coordinate (-) |
| ρbulk,cat | Bulk density of solid catalyst (g/L) |
| ρcat | Density of solid catalyst (g/L) |
| ζ | Dimensionless membrane area coordinate (-) |
| τ | NRTL binary interaction parameter (-) |
| Reactor voidage (volume of void/total reactor volume) | |
| Ω | Dimensionless membrane area/reactor volume ratio (-) |
| Superscripts | |
| e | Effective |
| i | Component |
| in | Input |
| j | Component |
| n | Total number of components |
| ° | Reference state/fresh feed |
| out | Output |
| P | Permeate |
| R | Retentate |
| Subscripts | |
| A | Limiting reactant |
| B | Reagents feed ratio |
| Cat | Catalyst |
| E | Ester |
| HAc | Acetic acid |
| i | Component |
| im | Component i flowing through the membrane section |
| k | Number of components |
| m | membrane |
| ROH | Isoamyl alcohol |
| r | Reactive retentate |
| p | Permeate |
| W | Water |
| Abbreviations | |
| HOC | Hayden-O’Connell |
| CSTR | Continuous Stirred Tank Reactor |
| FBR+PVMU | Fixed Bed Reactor and Pervaporation Membrane Unit |
| SR+PVMU | Slurry Reactor and Pervaporation Membrane Unit |
| IFBPVMR | Integrated Plug Flow Pervaporation Membrane Reactor |
| ISPVMR | Integrated Slurry and Pervaporation Membrane Reactor |
| NRTL | Non-Random Two Liquid |
| PFR | Plug Flow Reactor |
| PFPVMR | Plug Flow Pervaporation Membrane Reactor |
| PVMRs | Pervaporation membrane reactors |
References
- IEA. Renewables 2021; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Encyclopedia Britannica. Fusel Oil|Chemistry|Britannica. Available online: https://www.britannica.com/science/fusel-oil (accessed on 24 January 2022).
- Karimi, K.; Chisti, Y. Bioethanol Production and Technologies. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 273–284. [Google Scholar]
- IndustryARC. Isoamyl Acetate Market Share, Size and Industry Growth Analysis 2020–2025. Available online: https://www.industryarc.com/Research/Isoamyl-Acetate-Market-Research-502877 (accessed on 24 January 2022).
- López-Fernández, J.; Benaiges, M.D.; Sebastian, X.; Bueno, J.M.; Valero, F. Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts 2022, 12, 639. [Google Scholar] [CrossRef]
- Available online: https://www.seqens.com/en/products/isoamyl-acetate/ (accessed on 24 January 2022).
- Duque-Bernal, M.; Quintero-Arias, J.D.; Osorio-Viana, W.; Dobrosz- Gómez, I.; Fontalvo, J.; Gómez-García, M.A. Kinetic study on the homogeneous esterification of acetic acid with isoamyl alcohol. Int. J. Chem. Kinet. 2013, 45, 10–18. [Google Scholar] [CrossRef]
- Osorio-Viana, W.; Duque-Bernal, M.; Quintero-Arias, J.D.; Dobrosz-Gómez, I.; Fontalvo, J.; Gómez-García, M.Á. Activity model and consistent thermodynamic features for acetic acid-isoamyl alcohol-isoamyl acetate-water reactive system. Fluid Phase Equilibria 2013, 345, 68–80. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Gil, I.D.; Rodríguez, G. Fluid phase equilibria for the isoamyl acetate production by reactive distillation. Fluid Phase Equilibria 2020, 518, 112647. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Diban, N.; Aguayo, A.T.; Bilbao, J.; Urtiaga, A.; Ortiz, I. Membrane Reactors for in Situ Water Removal: A Review of Applications. Ind. Eng. Chem. Res. 2013, 52, 10342–10354. [Google Scholar] [CrossRef]
- Bruggen, B.V.; Luis, P. Pervaporation. In Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 101–154. [Google Scholar]
- Ugur Nigiz, F. A Comparative Study on the Synthesis of Ethyl Propionate in a Pervaporation Membrane Reactor. Chem. Eng. Process.-Process Intensif. 2018, 128, 173–179. [Google Scholar] [CrossRef]
- Kumar Purkait, M.; Randeep, S.; Piyal, M.; Dibyajyoti, H. Pervaporation. In Thermal Induced Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–120. [Google Scholar]
- Osorio-Viana, W.; Ibarra-Taquez, H.N.; Dobrosz-Gómez, I.; Gómez-García, M.Á. Hybrid membrane and conventional processes comparison for isoamyl acetate production. Chem. Eng. Proc. Process Intensif. 2014, 76, 70. [Google Scholar] [CrossRef]
- Zhu, Y.; Minet, R.G.; Tsotsis, T.T. A continuous pervaporation membrane reactor for the study of esterification reactions using a composite polymeric/ceramic membrane. Chem. Eng. Sci. 1996, 51, 4103–4113. [Google Scholar] [CrossRef]
- Sanz, M.T.; Gmehling, J. Esterification of acetic acid with isopropanol coupled with pervaporation Part I: Kinetics and pervaporation studies. Chem. Eng. J. 2006, 123, 1–8. [Google Scholar] [CrossRef]
- Sanz, M.T.; Gmehling, J. Esterification of acetic acid with isopropanol coupled with pervaporation Part II: Study of a pervaporation reactor. Chem. Eng. J. 2006, 123, 9–14. [Google Scholar] [CrossRef]
- Ugur Nigiz, F. Comparative study on use of pervaporation membrane reactor for lauric acid—Methanol esterification. Sep. Purif. Technol. 2021, 264, 118443. [Google Scholar] [CrossRef]
- Gómez-García, M.Á.; Dobrosz-Gómez, I.; Osorio-Viana, W. Experimental assessment and simulation of isoamyl acetate production using a batch pervaporation membrane reactor. Chem. Eng. Process.-Process Intensif. 2017, 122, 155–160. [Google Scholar] [CrossRef]
- Lim, S.Y.; Park, B.; Hung, F.; Sahimi, M.; Tsotsis, T.T. Design issues of pervaporation membrane reactors for esterification. Chem. Eng. Sci. 2002, 57, 4933–4946. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess function for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Osorio-Viana, W.; Duque-Bernal, M.; Dobrosz-Gómez, I.; Fontalvo, J.; Gómez-García, M.Á. Kinetic study on the catalytic esterification of acetic acid with isoamyl alcohol over Amberlite IR-120. Chem. Eng. Sci. 2013, 101, 755–763. [Google Scholar] [CrossRef]
- Osorio-Viana, W.; Quintero-Arias, J.D.; Dobrosz-Gómez, I.; Fontalvo, J.; Gómez-García, M.Á. Intensification of isoamyl acetate production -transport properties of silica membranes. Desalination Water Treat. 2013, 51, 2377–2386. [Google Scholar] [CrossRef]
- Hayden, J.G.; O’Connell, J.P. Generalized Method for Predicting Second Virial Coefficients. Ind. Eng. Chem. Process Des. Dev. 1975, 14, 209–216. [Google Scholar] [CrossRef]
- Gómez-García, M.Á.; Dobrosz-Gómez, I.; Rynkowski, J. Learning on chemical equilibrium shift assessment for membrane reactors using Gibbs free energy minimization method. Educ. Chem. Eng. 2018, 22, 20–26. [Google Scholar] [CrossRef]
- Available online: https://pervatech.com/products/hydrophilic-pervaporation-membranes (accessed on 24 January 2022).
- Patel, T.M.; Chheda, H.; Baheti, A.; Patel, P.; Nath, K. Comparative performance of flat sheet and spiral wound modules in the nanofiltration of reactive dye solution. Environ. Sci. Pollut. Res. Int. 2011, 19, 2994–3004. [Google Scholar] [CrossRef]
- Lau, H.S.; Lau, S.K.; Soh, L.S.; Hong, S.U.; Gok, X.Y.; Yi, S.; Yong, W.F. State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, J.; Pinelo, M.; Chen, G.; Wan, Y.; Luo, J. Modelling and optimization of pervaporation membrane modules: A critical review. J. Membr. Sci. 2022, 664, 121084. [Google Scholar] [CrossRef]
















| i | Water * | Water | Water | Acid | Acid | Alcohol |
|---|---|---|---|---|---|---|
| j | Acid * | Alcohol | Acetate | Alcohol | Acetate | Acetate |
| aij | 3.3293 | 1.5598 | 0.3194 | 0.4521 | −0.6992 | 0.1291 |
| aji | −1.9763 | −1.0959 | −2.5596 | 1.1307 | 0.0741 | 1.5083 |
| bij | −723.8881 | 1096.2001 | 1180.3569 | −335.9220 | 74.0710 | −229.6133 |
| bji | 609.8886 | 166.2337 | 996.9415 | −53.1049 | 440.9815 | −311.0556 |
| cij *,** | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 |
| Component | (m/h) | Bi (1/Bar) |
|---|---|---|
| Acetic acid | 1.56 × 10−6 | −35.365 |
| Isoamyl alcohol | 3.03 × 10−6 | −12.096 |
| Isoamyl acetate | 3.52 × 10−6 | 89.747 |
| Water | 9.60 × 10−4 | −2.951 |
| Dimensionless Number | Design Variables | Equation in Terms of Design Variables at 353 K | Expected Order of Magnitude |
|---|---|---|---|
| ** | ◊ ‡ | ◊ ‡ | |
| ◊ ‡ | ◊ ‡ | ||
| - | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Arias, J.D.; Dobrosz-Gómez, I.; de Lasa, H.; Gómez-García, M.-Á. Pervaporation Membrane-Catalytic Reactors for Isoamyl Acetate Production. Catalysts 2023, 13, 284. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020284
Quintero-Arias JD, Dobrosz-Gómez I, de Lasa H, Gómez-García M-Á. Pervaporation Membrane-Catalytic Reactors for Isoamyl Acetate Production. Catalysts. 2023; 13(2):284. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020284
Chicago/Turabian StyleQuintero-Arias, Jesús David, Izabela Dobrosz-Gómez, Hugo de Lasa, and Miguel-Ángel Gómez-García. 2023. "Pervaporation Membrane-Catalytic Reactors for Isoamyl Acetate Production" Catalysts 13, no. 2: 284. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020284