Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration
Abstract
:1. Introduction
2. Results and Discussion
2.1. BiOCl Characterization
2.2. Effect of Operating Parameters on LOS Degradation
2.3. Effect of Type of Irradiation on the Degradation of LOS
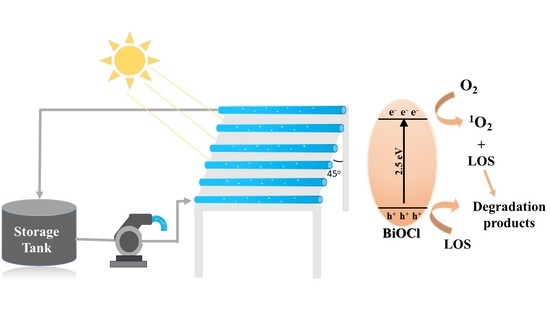
2.4. Photocatalytic Mechanism
2.5. Photocatalyst Reusability
2.6. Pilot-Plant Scale Photoreactor
3. Materials and Methods
3.1. Chemical and Water Matrices
3.2. Photocatalyst Synthesis and Characterization
- D represents the average crystallite size.
- λ corresponds to the wavelength of the X-rays used.
- β indicates the full width at half maximum (FWHM) of the diffraction peak.
- θ represents the diffraction angle.
- d represents the interplanar spacing.
- h, k, and l are the Miller indices.
3.3. Photoanode Fabrication
3.4. Photocatalytic Degradation Experiments
3.5. Chronoamperometric Measurements
3.6. Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging Organic Contaminants in Global Community Drinking Water Sources and Supply: A Review of Occurrence, Processes and Remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A Detailed Review on Advanced Oxidation Process in Treatment of Wastewater: Mechanism, Challenges and Future Outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx-BiVO4/SPS/Solar System. Catalysts 2022, 12, 882. [Google Scholar] [CrossRef]
- Fedorov, K.; Dinesh, K.; Sun, X.; Darvishi Cheshmeh Soltani, R.; Wang, Z.; Sonawane, S.; Boczkaj, G. Synergistic Effects of Hybrid Advanced Oxidation Processes (AOPs) Based on Hydrodynamic Cavitation Phenomenon—A Review. Chem. Eng. J. 2022, 432, 134191. [Google Scholar] [CrossRef]
- Antoniadou, M.; Falara, P.P.; Likodimos, V. Photocatalytic Degradation of Pharmaceuticals and Organic Contaminants of Emerging Concern Using Nanotubular Structures. Curr. Opin. Green Sustain. Chem. 2021, 29, 100470. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.; Makoś, P.; Wang, Z.; Boczkaj, G. Synergistic Effect of TiO2 Photocatalytic Advanced Oxidation Processes in the Treatment of Refinery Effluents. Chem. Eng. J. 2020, 391, 123488. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated Photocatalytic Advanced Oxidation System (TiO2/UV/O3/H2O2) for Degradation of Volatile Organic Compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Shah, N.S.; Khan, S.; Zhang, Y.; Boczkaj, G.; Khan, H.M.; Dionysiou, D.D. Synthesis of Eosin Modified TiO2 Film with Co-Exposed {001} and {101} Facets for Photocatalytic Degradation of Para-Aminobenzoic Acid and Solar H2 Production. Appl. Catal. B Environ. 2020, 265, 118557. [Google Scholar] [CrossRef]
- Monfort, O.; Pop, L.C.; Sfaelou, S.; Plecenik, T.; Roch, T.; Dracopoulos, V.; Stathatos, E.; Plesch, G.; Lianos, P. Photoelectrocatalytic Hydrogen Production by Water Splitting Using BiVO4 Photoanodes. Chem. Eng. J. 2016, 286, 91–97. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and Application of Bi2WO6 for the Photocatalytic Degradation of Two Typical Fluoroquinolones under Visible Light Irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, S.; Leghari, S.A.K.; Zhang, J. Nonstoichiometric Bi2O3: Efficient Visible Light Photocatalyst. RSC Adv. 2013, 3, 1363–1367. [Google Scholar] [CrossRef]
- Landge, V.K.; Sonawane, S.H.; Sivakumar, M.; Sonawane, S.S.; Uday Bhaskar Babu, G.; Boczkaj, G. S-Scheme Heterojunction Bi2O3-ZnO/Bentonite Clay Composite with Enhanced Photocatalytic Performance. Sustain. Energy Technol. Assess. 2021, 45, 101194. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, D.; Liu, C.; Zhou, X.; Yang, H.; Qu, J.; He, D. Efficient Photocatalytic Water Disinfection by a Novel BP/BiOBr S-Scheme Heterojunction Photocatalyst. Chem. Eng. J. 2023, 468, 143581. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Harunsani, M.H. Ag3PO4 and Ag3PO4–Based Visible Light Active Photocatalysts: Recent Progress, Synthesis, and Photocatalytic Applications. Catal. Commun. 2022, 172, 106556. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Chen, G.; Wang, S.; Yao, S. Inducing Photocarrier Separation via 3D Porous Faveolate Cross-Linked Carbon to Enhance Photothermal/Pyroelectric Property. Adv. Powder Mater. 2022, 1, 100032. [Google Scholar] [CrossRef]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent Advances in Bismuth-Based Photocatalysts: Environment and Energy Applications. Green Energy Environ. 2022; in press. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Zhu, W.; Yang, X.; Luo, Y. TBAOH Assisted Synthesis of Ultrathin BiOCl Nanosheets with Enhanced Charge Separation Efficiency for Superior Photocatalytic Activity in Carbamazepine Degradation. J. Colloid Interface Sci. 2020, 570, 242–250. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Fan, M.; Tong, P.; Sun, J.; Dong, S.; Sun, J. Ultra-Light and Compressible 3D BiOCl/RGO Aerogel with Enriched Synergistic Effect of Adsorption and Photocatalytic Degradation of Oxytetracycline. J. Mater. Res. Technol. 2019, 8, 4577–4587. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Mehmood, R.; Athar, M.; Hussain, J.; Wei, Y.; Khaliq, A. BiOCl Nanoplates Doped with Fe3+ Ions for the Visible-Light Degradation of Aqueous Pollutants. ACS Appl. Nano Mater. 2021, 4, 746–758. [Google Scholar] [CrossRef]
- Petala, A.; Arvaniti, O.S.; Travlou, G.; Mantzavinos, D.; Frontistis, Z. Solar Light Induced Photocatalytic Removal of Sulfamethoxazole from Water and Wastewater Using BiOCl Photocatalyst. J. Environ. Sci. Health Part A 2021, 56, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Grilla, E.; Kagialari, M.N.; Petala, A.; Frontistis, Z.; Mantzavinos, D. Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions. Catalysts 2021, 11, 650. [Google Scholar] [CrossRef]
- Galani, A.; Alygizakis, N.; Aalizadeh, R.; Kastritis, E.; Dimopoulos, M.-A.; Thomaidis, N.S. Patterns of Pharmaceuticals Use during the First Wave of COVID-19 Pandemic in Athens, Greece as Revealed by Wastewater-Based Epidemiology. Sci. Total Environ. 2021, 798, 149014. [Google Scholar] [CrossRef]
- Salazar, C.; Contreras, N.; Mansilla, H.D.; Yáñez, J.; Salazar, R. Electrochemical Degradation of the Antihypertensive Losartan in Aqueous Medium by Electro-Oxidation with Boron-Doped Diamond Electrode. J. Hazard. Mater. 2016, 319, 84–92. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.-C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of Drug Losartan in Environmental Aquatic Matrices by Heat-Activated Persulfate: Kinetics, Transformation Products and Synergistic Effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Vieira, M.G.A.; da Silva, M.G.C.; Wang, S. Oxidative Degradation of Pharmaceutical Losartan Potassium with N-Doped Hierarchical Porous Carbon and Peroxymonosulfate. Chem. Eng. J. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Silva-Agredo, J.; Ávila-Torres, Y.; Torres-Palma, R.A. Dataset on the Degradation of Losartan by TiO(2)-Photocatalysis and UVC/Persulfate Processes. Data Br. 2020, 31, 105692. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Zhu, W.; Luo, Y. Controllable Synthesis of Solar-Light-Driven BiOCl Nanostructures for Highly Efficient Photocatalytic Degradation of Carbamazepine. J. Solid State Chem. 2019, 277, 133–138. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C. Photocatalytic Degradation of Carbamazepine Using Hierarchical BiOCl Microspheres: Some Key Operating Parameters, Degradation Intermediates and Reaction Pathway. Chem. Eng. J. 2015, 273, 156–165. [Google Scholar] [CrossRef]
- Shen, T.; Shi, X.; Guo, J.; Li, J.; Yuan, S. Photocatalytic Removal of NO by Light-Driven Mn3O4/BiOCl Heterojunction Photocatalyst: Optimization and Mechanism. Chem. Eng. J. 2021, 408, 128014. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.K.; Hussain, M. Efficient Visible Light Assisted Photocatalysis Using ZnO/TiO2 Nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Kollipara, S.; Bende, G.; Bansal, Y.; Saha, R. Stability-Indicating Reversed-Phase Liquid Chromatographic Method for Simultaneous Determination of Losartan Potassium and Ramipril in Tablets. Indian J. Pharm. Sci. 2012, 74, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapińska, D.; Adamek, E.; Masternak, E.; Zielińska-Danch, W.; Baran, W. Influence of PH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions. Toxics 2022, 10, 655. [Google Scholar] [CrossRef]
- Pusceddu, F.H.; Guimarães, M.M.; Lopes, L.O.; Souza, L.S.; Cortez, F.S.; Pereira, C.D.S.; Choueri, R.B.; Cesar, A. Biological Effects of the Antihypertensive Losartan under Different Ocean Acidification Scenarios. Environ. Pollut. 2022, 292, 118329. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of Water Matrix on Photocatalytic Degradation and General Kinetic Modeling. Appl. Catal. B Environ. 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Duta, A.; Malato, S.; Bogatu, C.; Covei, M.; Polo-l, M.I. Novel ZnO Photocatalysts for Pollutants’ Abatement under Solar Radiation at Pilot Plant Scale. Catal. Today 2023, 415, 113947. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, B.; Wang, F.; Yuan, R.; Chen, H.; Han, X. Effect of Dissolved Organic Matters and Inorganic Ions on TiO2 Photocatalysis of Diclofenac: Mechanistic Study and Degradation Pathways. Environ. Sci. Pollut. Res. 2020, 27, 2044–2053. [Google Scholar] [CrossRef]
- Metheniti, M.; Frontistis, Z.; Ribeiro, R.; Silva, A.; Faria, J.; Gomes, H.; Mantzavinos, D. Degradation of Propyl Paraben by Activated Persulfate Using Iron-Containing Magnetic Carbon Xerogels: Investigation of Water Matrix and Process Synergy Effects. Environ. Sci. Pollut. Res. 2018, 25, 34801–34810. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.P.; Hsu, M.H.; Lin, A.Y.C. The Role of Bicarbonate Anions in Methotrexate Degradation via UV/TiO2: Mechanisms, Reactivity and Increased Toxicity. Water Res. 2017, 112, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of Photocatalysis and Their Coping Strategies. Chem Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Petala, A.; Tsikritzis, D.; Kollia, M.; Ladas, S.; Kennou, S.; Kondarides, D.I. Synthesis and Characterization of N-Doped TiO2 Photocatalysts with Tunable Response to Solar Radiation. Appl. Surf. Sci. 2014, 305, 281–291. [Google Scholar] [CrossRef]
- Sarwan, B.; Pare, B.; Acharya, A.D.; Jonnalagadda, S.B. Mineralization and Toxicity Reduction of Textile Dye Neutral Red in Aqueous Phase Using BiOCl Photocatalysis. J. Photochem. Photobiol. B Biol. 2012, 116, 48–55. [Google Scholar] [CrossRef]
- Schneider, T.; Peralta-zamora, P.; Firak, D.S.; Ribeiro, R.R. Use of Scavenger Agents in Heterogeneous Photocatalysis: Truths, Half-Truths, and Misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Bancirova, M. Sodium Azide as a Specific Quencher of Singlet Oxygen during Chemiluminescent Detection by Luminol and Cypridina Luciferin Analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef]
- Yang, B.J.; Luo, W.; Liao, Q.; Zhu, J.Y.; Gan, M.; Liu, X.D.; Qiu, G.Z. Photogenerated-Hole Scavenger for Enhancing Photocatalytic Chalcopyrite Bioleaching. Trans. Nonferrous Met. Soc. China 2020, 30, 200–211. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Comment on “Singlet Oxygen 1O2 in Photocatalysis on TiO2. Where Does It Come From?” J. Phys. Chem. C 2019, 123, 27993–27995. [Google Scholar] [CrossRef] [Green Version]
- Barrios, B.; Mohrhardt, B.; Doskey, P.V.; Minakata, D. Mechanistic Insight into the Reactivities of Aqueous-Phase Singlet Oxygen with Organic Compounds. Environ. Sci. Technol. 2021, 55, 8054–8067. [Google Scholar] [CrossRef] [PubMed]
- Ioannidi, A.A.; Zappa, J.; Petala, A.; Souliotis, M.; Mantzavinos, D.; Frontistis, Z. Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate. Water 2023, 15, 1370. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and Disinfection of Water by Solar Photocatalysis: The Pilot Plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Hou, J.; Dai, D.; Wei, R.; Wu, X.; Wang, X.; Tahir, M.; Zou, J.-J. Narrowing the Band Gap of BiOCl for the Hydroxyl Radical Generation of Photocatalysis under Visible Light. ACS Sustain. Chem. Eng. 2019, 7, 16569–16576. [Google Scholar] [CrossRef]
- Yahia, I.S.; Jilani, A.; Abdel-wahab, M.S.; Zahran, H.Y. Optik The Photocatalytic Activity of Graphene Oxide/Ag3PO4 Nano-Composite: Loading Effect. Optik 2016, 127, 10746–10757. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ganesh, V.; Ravi Kumar, B.; AlAbdulaal, T.H.; Yahia, I.S.; Abdel-wahab, M.S.; Ade, R.; Hussien, M.S.A.; Keshway, M. Electrocatalytic Degradation of Rhodamine B Using Li-Doped ZnO Nanoparticles: Novel Approach. Materials 2023, 16, 1177. [Google Scholar] [CrossRef]
- Abdel-wahab, M.S. Substrate Temperature Impact on the Structural, Optical and Photo-Catalytic Activity of Sputtered Cu-Doped ZnO Thin Films. J. Electron. Mater. 2021, 50, 4364–4372. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yang, B.; Wang, B.; Chen, J.; Jing, H. In Situ Grown Heterojunction of Bi2WO6/BiOCl for Efficient Photoelectrocatalytic CO2 Reduction. J. Catal. 2019, 377, 209–217. [Google Scholar] [CrossRef]












| Parameters | Values (Unit) |
|---|---|
| Lattice constant (a = b) | 2.75 |
| Lattice constant (c) | 7.39 |
| Unit cell volume (V) | 55. 9 |
| Average crystallite size (D) | 32.2 nm |
| 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouvelis, K.; Ioannidi, A.A.; Petala, A.; Souliotis, M.; Frontistis, Z. Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration. Catalysts 2023, 13, 1175. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13081175
Kouvelis K, Ioannidi AA, Petala A, Souliotis M, Frontistis Z. Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration. Catalysts. 2023; 13(8):1175. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13081175
Chicago/Turabian StyleKouvelis, Konstantinos, Alexandra A. Ioannidi, Athanasia Petala, Manolis Souliotis, and Zacharias Frontistis. 2023. "Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration" Catalysts 13, no. 8: 1175. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13081175