Improved Hydrothermal Stability in Glass Diesel Soot Oxidation Catalysts
Abstract
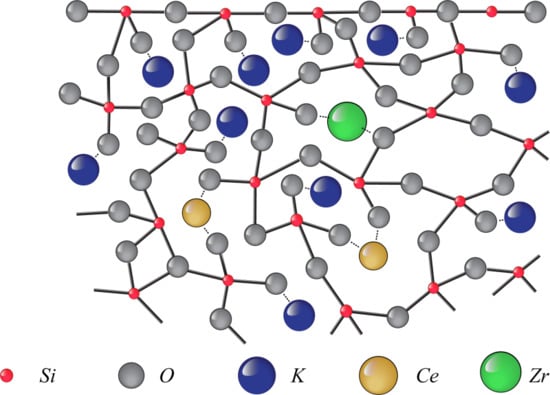
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Characterization by HR-TGA
2.2. Hydrothermal Testing of KCeSZ-1 Glass Composition
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- di Sarli, V.; Landi, G.; Lisi, L.; di Benedetto, A. Ceria-Coated Diesel Particulate Filters for Continuous Regeneration. AICHE J. 2017, 63, 3442–3449. [Google Scholar] [CrossRef]
- Yuan, S.B.; Meriaudeau, P.; Perrichon, V. Catalytic Combustion of Diesel Soot Particles on Copper—Catalysts Supported on TiO2—Effect of Potassium Promoter on the Activity. Appl. Catal. B Environ. 1994, 3, 319–333. [Google Scholar] [CrossRef]
- Serra, V.; Saracco, G.; Badini, C.; Specchia, V. Combustion of carbonaceous materials by Cu-K-V based catalysts: 2. Reaction mechanism. Appl. Catal. B Environ. 1997, 11, 329–346. [Google Scholar] [CrossRef]
- Querini, C.A.; Ulla, M.A.; Requejo, F.; Soria, J.; Sedran, U.A.; Miro, E.E. Catalytic combustion of diesel soot particles. Activity and characterization of Co/MgO and Co,K/MgO catalysts. Appl. Catal. B Environ. 1998, 15, 5–19. [Google Scholar] [CrossRef]
- Teraoka, Y.; Kanada, K.; Kagawa, S. Synthesis of La-K-Mn-O perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates. Appl. Catal. B Environ. 2001, 34, 73–78. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.M.; Duan, A.; Zhu, L.; Wang, X.Z. Diesel soot oxidation over supported vanadium oxide and K-promoted vanadium oxide catalysts. Appl. Catal. B Environ. 2005, 61, 36–46. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Diesel soot combustion activity of ceria promoted with alkali metals. Catal. Today 2008, 136, 3–10. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, H.; Xin, Y.; Tian, G.K.; Lu, C.X.; Zhang, Z.L.; Zheng, L.R.; Zheng, L. K-supported catalysts for diesel soot combustion: Making a balance between activity and stability. Catal. Today 2016, 264, 171–179. [Google Scholar] [CrossRef]
- Liu, T.Z.; Li, Q.; Xin, Y.; Zhang, Z.L.; Tang, X.F.; Zheng, L.R.; Gao, P.X. Quasi free K cations confined in hollandite-type tunnels for catalytic solid (catalyst)-solid (reactant) oxidation reactions. Appl. Catal. B Environ. 2018, 232, 108–116. [Google Scholar] [CrossRef]
- Jakubek, T.; Kaspera, W.; Legutko, P.; Stelmachowski, P.; Kotarba, A. How to Efficiently Promote Transition Metal Oxides by Alkali towards Catalytic Soot Oxidation. Top. Catal. 2016, 59, 1083–1089. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Strong Enhancement of deSoot Activity of Transition Metal Oxides by Alkali Doping: Additive Effects of Potassium and Nitric Oxide. Top. Catal. 2017, 60, 162–170. [Google Scholar] [CrossRef]
- Meloni, E.; Palma, V.; Vaiano, V. Optimized microwave susceptible catalytic diesel soot trap. Fuel 2017, 205, 142–152. [Google Scholar] [CrossRef]
- Rinkenburger, A.; Toriyama, T.; Yasuda, K.; Niessner, R. Catalytic Effect of Potassium Compounds in Soot Oxidation. ChemCatChem 2017, 9, 3513–3525. [Google Scholar] [CrossRef]
- Janiak, C.; Hoffmann, R.; Sjovall, P.; Kasemo, B. The Potassium Promoter Function in the Oxidation of Graphite—An Experimental and Theoretical-Study. Langmuir 1993, 9, 3427–3440. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Speechia, V. The role of suprafacial oxygen in some perovskites for the catalytic combustion of soot. J. Catal. 2003, 217, 367–375. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Xin, Y.; Zhang, Z.L.; Zhang, Y.X.; Hao, C.; Meng, M.; Zheng, L.R.; Zheng, L. A unified intermediate and mechanism for soot combustion on potassium-supported oxides. Sci. Rep. 2014, 4, 4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelles, S.J.; van Setten, B.; Makkee, M.; Moulijn, J.A. Molten salts as promising catalysts for oxidation of diesel soot: Importance of experimental conditions in testing procedures. Appl. Catal. B Environ. 1999, 21, 35–49. [Google Scholar] [CrossRef]
- McKee, D.W.; Chatterji, D. Catalytic Behavior of Alkali-Metal Carbonates and Oxides in Graphite Oxidation Reactions. Carbon 1975, 13, 381–390. [Google Scholar] [CrossRef]
- McKee, D.W. Gasification of Graphite in Carbon-Dioxide and Water-Vapor—The Catalytic Effects of Alkali-Metal Salts. Carbon 1982, 20, 59–66. [Google Scholar] [CrossRef]
- Kimura, R.; Elangovan, S.P.; Ogura, M.; Ushiyama, H.; Okubo, T. Alkali Carbonate Stabilized on Aluminosilicate via Solid Ion Exchange as a Catalyst for Diesel Soot Combustion. J. Phys. Chem. C 2011, 115, 14892–14898. [Google Scholar] [CrossRef]
- Grzona, C.B.; Lick, I.D.; Castellon, E.R.; Ponzi, M.I.; Ponzi, E.N. Cobalt and KNO3 supported on alumina catalysts for diesel soot combustion. Mater. Chem. Phys. 2010, 123, 557–562. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tokubuchi, N.; Arita, M.; Inoue, M.; Mochida, I. Catalytic combustion of carbon by alkali metal carbonates supported on perovskite-type oxide. Energy Fuels 1997, 11, 832–836. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nakano, K.; Kagawa, S.; Shangguan, W.F. Simultaneous Removal of Nitrogen-Oxides and Diesel Soot Particulates Catalyzed by Perovskite-Type Oxides. Appl. Catal. B Environ. 1995, 5, L181–L185. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagawa, S. Promotion effect of potassium on the catalytic property of CuFe2O4 for the simultaneous removal of NOx and diesel soot particulate. Appl. Catal. B Environ. 1998, 16, 149–154. [Google Scholar] [CrossRef]
- Milt, V.G.; Pissarello, M.L.; Miro, E.E.; Querini, C.A. Abatement of diesel-exhaust pollutants: NOx storage and soot combustion on K/La2O3 catalysts. Appl. Catal. B Environ. 2003, 41, 397–414. [Google Scholar] [CrossRef]
- Hizbullah, K.; Kureti, S.; Weisweiler, W. Potassium promoted iron oxide catalysts for simultaneous catalytic removal of nitrogen oxides and soot from diesel exhaust gas. Catal. Today 2004, 93–95, 839–843. [Google Scholar] [CrossRef]
- Peralta, M.A.; Milt, V.G.; Cornaglia, L.M.; Querini, C.A. Stability of Ba,K/CeO2 catalyst during diesel soot combustion: Effect of temperature, water, and sulfur dioxide. J. Catal. 2006, 242, 118–130. [Google Scholar] [CrossRef]
- Moggia, J.M.; Milt, V.G.; Ulla, M.A.; Cornaglia, L.M. Surface characterization of Co,K/La2O3 catalysts used for the catalytic combustion of diesel soot. Surf. Interface Anal. 2003, 35, 216–225. [Google Scholar] [CrossRef]
- An, H.M.; Su, C.S.; McGinn, P.J. Application of potash glass as a catalyst for diesel soot oxidation. Catal. Commun. 2009, 10, 509–512. [Google Scholar] [CrossRef]
- Su, C.S.; McGinn, P.J. Application of glass soot catalysts on metal supports to achieve low soot oxidation temperature. Catal. Commun. 2014, 43, 1–5. [Google Scholar] [CrossRef]
- Zokoe, J.; McGinn, P.J. Catalytic diesel soot oxidation by hydrothermally stable glass catalysts. Chem. Eng. J. 2015, 262, 68–77. [Google Scholar] [CrossRef]
- Zokoe, J.; Su, C.; McGinn, P.J. Soot Combustion Activity and Potassium Mobility in Diesel Particulate Filters Coated with a K–Ca–Si–O Glass Catalyst. Ind. Eng. Chem. Res. 2019, 58, 11891–11901. [Google Scholar] [CrossRef]
- Su, C.S.; McGinn, P.J. The effect of Ca2+ and Al3+ additions on the stability of potassium disilicate glass as a soot oxidation catalyst. Appl. Catal. B Environ. 2013, 138, 70–78. [Google Scholar] [CrossRef]
- Lopez-Suarez, F.E.; Bueno-Lopez, A.; Illan-Gomez, M.J.; Ura, B.; Trawczynski, J. Potassium Stability in Soot Combustion Perovskite Catalysts. Top. Catal. 2009, 52, 2097–2100. [Google Scholar] [CrossRef]
- Neyertz, C.A.; Miro, E.E.; Querini, C.A. K/CeO2 catalysts supported on cordierite monoliths: Diesel soot combustion study. Chem. Eng. J. 2012, 181, 93–102. [Google Scholar] [CrossRef]
- White, W.B. Theory of Corrosion of Glass and Ceramics. In Corrosion of Glass, Ceramics, and Ceramic Superconductors: Principles, Testing, Characterization and Applications; Clark, D.E., Zoitos, B.K., Eds.; Noyes Publications: Park Ridge, NJ, USA, 1992; pp. 2–28. [Google Scholar]
- Perret, D.; Crovisier, J.L.; Stille, P.; Shields, G.; Mader, U.; Advocat, T.; Schenk, K.; Chardonnens, M. Thermodynamic stability of waste glasses compared to leaching behavior. Appl. Geochem. 2003, 18, 1165–1184. [Google Scholar] [CrossRef]
- Silva, A.C.; Mello-Castanho, S.R.H. Vitrified galvanic waste chemical stability. J. Eur. Ceram. Soc. 2007, 27, 565–570. [Google Scholar] [CrossRef]
- Melcher, M.; Wiesinger, R.; Schreiner, M. Degradation of Glass Artifacts: Application of Modern Surface Analytical Techniques. Acc. Chem. Res. 2010, 43, 916–926. [Google Scholar] [CrossRef]
- Newton, R.G. The Durability of Glass—A Review. Glass Technol. 1985, 26, 21–38. [Google Scholar]
- Gentaz, L.; Lombardo, T.; Loisel, C.; Chabas, A.; Vallotto, M. Early stage of weathering of medieval-like potash-lime model glass: Evaluation of key factors. Environ. Sci. Pollut. Res. 2011, 18, 291–300. [Google Scholar] [CrossRef]
- An, H.M.; Kilroy, C.; McGinn, P.J. Combinatorial synthesis and characterization of alkali metal doped oxides for diesel soot combustion. Catal. Today 2004, 98, 423. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tokubuchi, N.; Inoue, M.; Arita, M.; Mochida, I. Catalytic activities of K2CO3 supported on several oxides for carbon combustion. Energy Fuels 1998, 12, 870–874. [Google Scholar] [CrossRef]
- Paul, A. Chemical Durability of Glasses—Thermodynamic Approach. J. Mater. Sci. 1977, 12, 2246–2268. [Google Scholar] [CrossRef]
- Jantzen, C.M. Thermodynamic Approach to Glass Corrosion. In Corrosion of Glass, Ceramics, and Ceramic Superconductors: Principles, Testing, Characterization and Applications; Clark, D.E., Zoitos, B.K., Eds.; Noyes Publications: Park Ridge, NJ, USA, 1992; pp. 153–217. [Google Scholar]
- McKee, D.W. The Catalyzed Gasification Reactions of Carbon. Chem. Phys. Carbon 1981, 16, 1–118. [Google Scholar]
- Sato, K.; Yamaguchi, M.; Fujita, S.; Suzuki, K.; Mori, T. Enhancement of the activity of calcium aluminosilicate (Ca12Al10Si4O35) for the combustion of diesel soot via the substitution of Ca2+ ions with transition metal ions. Catal. Commun. 2006, 7, 132–135. [Google Scholar] [CrossRef]
- Das, C.R. Chemical Durability of Sodium-Silicate Glasses Containing Al2O3 and ZrO2. J. Am. Ceram. Soc. 1981, 64, 188–193. [Google Scholar] [CrossRef]
- Karell, R.; Kraxner, J.; Chromcikova, M. Properties of selected, zirconia containing silicate glasses. Ceram. Silik. 2006, 50, 78–82. [Google Scholar]
- Stanova, I.; Plsko, A.; Pagacova, J.; Sibikova, K. Influence of composition on corroding process of Na2O-K2O-CaO-ZrO2-SiO2 glasses. Chem. Pap. 2007, 61, 11–15. [Google Scholar] [CrossRef]
- Cailleteau, C.; Angeli, F.; Devreux, F.; Gin, S.; Jestin, J.; Jollivet, P.; Spalla, O. Insight into silicate-glass corrosion mechanisms. Nat. Mater. 2008, 7, 978–983. [Google Scholar] [CrossRef]
- Sajeevan, A.C.; Sajith, V. A study on Oxygen Storage capacity of Zirconium-Cerium-Oxide Nanoparticles. In Advanced Materials Research Iii; Gupta, K.M., Ed.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; pp. 123–127. [Google Scholar]
- Zhang, J.; Kumagai, H.; Yamamura, K.; Ohara, S.; Takami, S.; Morikawa, A.; Shinjoh, H.; Kaneko, K.; Adschiri, T.; Suda, A. Extra-Low-Temperature Oxygen Storage Capacity of CeO2 Nanocrystals with Cubic Facets. Nano Lett. 2011, 11, 361–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Andersson, S.; Muhammed, M. Nanophase Catalytic Oxides. 1. Synthesis of Doped Cerium Oxides as Oxygen Storage Promoters. Appl. Catal. B Environ. 1995, 6, 325–337. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. CeO2-based catalysts with engineered morphologies for soot oxidation to enhance soot-catalyst contact. Nanoscale Res. Lett. 2014, 9, 254. [Google Scholar] [CrossRef]
- Tomozawa, M.; Kim, D.L.; Agarwal, A.; Davis, K.M. Water diffusion and surface structural relaxation of silica glasses. J. Non-Cryst. Solids 2001, 288, 73–80. [Google Scholar] [CrossRef]
- Ingram, M.D.; Wu, M.H.; Coats, A.; Kamitsos, E.I.; Varsamis, C.P.E.; Garcia, N.; Sola, M. Evidence from infrared spectroscopy of structural relaxation during field assisted and chemically driven ion exchange in soda-lime-silica glasses. Phys. Chem. Glasses 2005, 46, 84–89. [Google Scholar]
- Koike, A.; Tomozawa, M. Towards the origin of the memory effect in oxide glasses. J. Non-Cryst. Solids 2008, 354, 3246–3253. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Diesel particulate emission control. Fuel Process. Technol. 1996, 47, 1–69. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamauchi, K. Numerical simulation of continuously regenerating diesel particulate filter. Proc. Combust. Inst. 2013, 34, 3083–3090. [Google Scholar] [CrossRef]
- ElBatal, H.A.; Azooz, M.A.; Khalil, E.M.A.; Monem, A.S.; Hamdy, Y.M. Characterization of some bioglass-ceramics. Mater. Chem. Phys. 2003, 80, 599–609. [Google Scholar] [CrossRef]
- Vilarigues, M.; da Silva, R.C. Characterization of potash-glass corrosion in aqueous solution by ion beam and IR spectroscopy. J. Non-Cryst. Solids 2006, 352, 5368–5375. [Google Scholar] [CrossRef]
- Vilarigues, M.; da Silva, R.C. The effect of Mn, Fe and Cu ions on potash-glass corrosion. J. Non-Cryst. Solids 2009, 355, 1630–1637. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.D.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Mahamulkar, S.; Yin, K.H.; Agrawal, P.K.; Davis, R.J.; Jones, C.W.; Malek, A.; Shibata, H. Formation and Oxidation/Gasification of Carbonaceous Deposits: A Review. Ind. Eng. Chem. Res. 2016, 55, 9760–9818. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Wu, X.D.; Liu, D.X.; Li, K.; Li, J.; Weng, D. Role of CeO2-ZrO2 in diesel soot oxidation and thermal stability of potassium catalyst. Catal. Commun. 2007, 8, 1274–1278. [Google Scholar] [CrossRef]
- Alinezhadchamazketi, A.; Khodadadi, A.A.; Mortazavi, Y.; Nemati, A. Catalytic evaluation of promoted CeO2-ZrO2 by transition, alkali, and alkaline-earth metal oxides for diesel soot oxidation. J. Environ. Sci. 2013, 25, 2498–2506. [Google Scholar] [CrossRef]
- Neyertz, C.A.; Banus, E.D.; Miro, E.E.; Querini, C.A. Potassium-promoted Ce0.65Zr0.35O2 monolithic catalysts for diesel soot combustion. Chem. Eng. J. 2014, 248, 394–405. [Google Scholar] [CrossRef]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of Catalase Mimetic Activity in Ce3+/Ce4+ Doped Bioactive Glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef]
- Nicolini, V.; Varini, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P. The effect of composition on structural, thermal, redox and bioactive properties of Ce-containing glasses. Mater. Des. 2016, 97, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Nicolini, V.; Malavasi, G.; Menabue, L.; Lusvardi, G.; Benedetti, F.; Valeri, S.; Luches, P. Cerium-doped bioactive 45S5 glasses: Spectroscopic, redox, bioactivity and biocatalytic properties. J. Mater. Sci. 2017, 52, 8845–8857. [Google Scholar] [CrossRef]
- Benedetti, F.; Luches, P.; D’Addato, S.; Valeri, S.; Nicolini, V.; Pedone, A.; Menziani, M.C.; Malavasi, G. Structure of active cerium sites within bioactive glasses. J. Am. Ceram. Soc. 2017, 100, 5086–5095. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and characterization of cerium-doped glasses and in vitro evaluation of bioactivity. J. Non-Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Su, C.S. Stabilization of potassium in soot oxidation catalysts and their application on diesel particulate filters. In Chemical & Biomolecular Engineering; University of Notre Dame: Notre Dame, IN, USA, 2011. [Google Scholar]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non-Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Wellbrock, U.; Beier, W.; Frischat, G.H. Preparation of SiO2-TiO2-ZrO2 Gel Glasses and Coatings By Means Of Modified Alkoxide Solutions. J. Non-Cryst. Solids 1992, 147, 350–355. [Google Scholar] [CrossRef]
- Avendano, R.G.R.; de los Reyes, J.A.; Montoya, J.A.; Viveros, T. Effect of synthesis parameters on sol-gel silica modified by zirconia. J. Sol-Gel Sci. Technol. 2005, 33, 133–138. [Google Scholar] [CrossRef]
- Su, C.; Wang, Y.; Kumar, A.; McGinn, P. Simulating Real World Soot-Catalyst Contact Conditions for Lab-Scale Catalytic Soot Oxidation Studies. Catalysts 2018, 8, 247. [Google Scholar] [CrossRef]
- Resitoglu, I.A.; Altinisik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]




| Composition | Contact Condition | |||
|---|---|---|---|---|
| Tight | Loose | |||
| Tig | T50 | Tig | T50 | |
| KCS-1 | 367.16 | 376.29 | 382.76 | 405.06 |
| KCeZr-1 | 365.1 | 372.36 | 395.91 | 410.67 |
| KCeZr-2 | 363.72 | 370.81 | 392.8 | 402.32 |
| KCeZr-3 | 369.79 | 374.21 | 399.18 | 411.23 |
| KCS-1 | KCeSZ-1 | |||
|---|---|---|---|---|
| Hydrothermal Temperature 2 h Exposure | Tig (°C) | T50 (°C) | Tig (°C) | T50 (°C) |
| As-Made | 388 | 405 | 390 | 405 |
| 300 | 368 | 380 | 359 | 384 |
| 500 | 387 | 401 | 388 | 401 |
| 600 | 413 | 430 | 391 | 406 |
| 700 | 425 | 468 | 410 | 427 |
| Composition | Si | K | Ca | Ce | Zr | ΔGhyd (kJ/mol) |
|---|---|---|---|---|---|---|
| KCS-1 | 47 | 40.4 | 12.6 | 0 | 0 | −40.4 |
| KCeSZ-1 | 45 | 45 | 0 | 8 | 2 | −31.6 |
| KCeSZ-2 | 43.7 | 44.7 | 0 | 9.71 | 1.94 | −31.90 |
| KCeSZ-3 | 42.06 | 44.9 | 0 | 11.2 | 1.87 | −34.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zokoe, J.; Feng, X.; Su, C.; McGinn, P.J. Improved Hydrothermal Stability in Glass Diesel Soot Oxidation Catalysts. Catalysts 2019, 9, 684. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080684
Zokoe J, Feng X, Su C, McGinn PJ. Improved Hydrothermal Stability in Glass Diesel Soot Oxidation Catalysts. Catalysts. 2019; 9(8):684. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080684
Chicago/Turabian StyleZokoe, James, Xiaoxiang Feng, Changsheng Su, and Paul J. McGinn. 2019. "Improved Hydrothermal Stability in Glass Diesel Soot Oxidation Catalysts" Catalysts 9, no. 8: 684. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080684