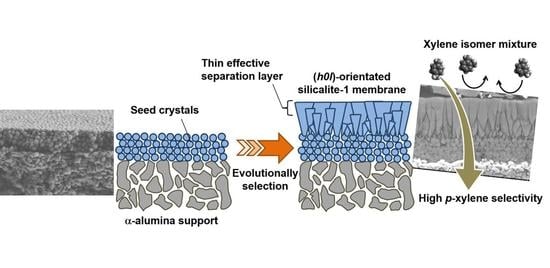
Formation Process of Columnar Grown (101)-Oriented Silicalite-1 Membrane and Its Separation Property for Xylene Isomer
Abstract
:1. Introduction
2. Experimental
2.1. Preparations of Seed Crystal and Seeded Support
2.2. Membrane Preparation
2.3. Microscopic Observations
2.4. N2 Adsorption Measurement
2.5. Nano-Permporometry Test
2.6. Permeation Test
3. Results and Discussion
3.1. Microscopic Observation of Membrane Formation
3.2. Changes of Crystallinity and Orientation
3.3. Molecular Sieving Properties and Defect Amount
3.4. Xylene Isomer Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Kanezashi, M.; Shazwani, W.N.; Yoshioka, T.; Tsuru, T. Separation of propylene/propane binary mixtures by bis(triethoxysilyl) methane (BTESM)-dericed silica membranes fabricated at different calcination temperatures. J. Membr. Sci. 2012, 415–416, 478–485. [Google Scholar] [CrossRef]
- Ikeda, A.; Nomura, M. Preparation of Amorphous Silica Based Membranes for Separation of Hydrocarbons. J. Jpn. Petrol. Inst. 2016, 59, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J. Membr. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Hayashi, J.; Mizuta, H.; Yamamoto, M.; Kusakabe, K.; Morooka, S.; Suh, S.-H. Separation of Ethane/Ethylene and Propane/Propylene Systems with a Carbonized BPDA-pp’ODA Polyimide Membrane. Ind. Eng. Chem. Res. 1996, 35, 4176–4181. [Google Scholar] [CrossRef]
- Hedlund, J.; Sterte, J.; Anthonis, M.; Bons, A.-J.; Carstensen, B.; Corcoran, N.; Cox, D.; Deckman, H.; Gijnst, W.D.; Moor, P.-P.; et al. High-flux MFI membranes. Microporous Mesoporous Mater. 2002, 52, 179–189. [Google Scholar] [CrossRef]
- Funke, H.H.; Argo, A.M.; Falconer, J.L.; Noble, R.D. Separations of Cyclic, Branched, and Linear Hydrocarbon Mixtures through Silicalite Membranes. Ind. Eng. Chem. Res. 1997, 334, 137–143. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Lombardo, E.A. Influence of exchanged cations (Na+, Cs+, Sr2+ and Ba2+) on xylene permeation through ZSM-5/SS tubular membranes. Sep. Purif. Technol. 2008, 61, 136–147. [Google Scholar] [CrossRef]
- Lai, Z.; Bonilla, G.; Diaz, I.; Nery, J.G.; Sujaoti, K.; Amat, M.A.; Kokkoli, E.; Terasaki, O.; Thompson, R.W.; Tsapatsis, M.; et al. Microstructural Optimization of a Zeolite Membrane for Organic Vapor Separation. Science 2003, 300, 456–460. [Google Scholar]
- Lai, Z.; Tsapatsis, M.; Nicolich, J.P. Siliceous ZSM-5 Membranes by Secondary Growth of b-Oriented Seed Layers. Adv. Funct. Mater. 2004, 14, 716–729. [Google Scholar] [CrossRef]
- Daramola, M.O.; Burger, A.J.; Giroir-Fendler, A.; Miachon, S.; Lorenzen, L. Extractor-type catalytic membrane reactor with nanocomposite MFI-alumina membrane tube as separation unit: Prospect for ultra-pure para-Xylene production from m-Xylene isomerization over Pt-HZSM-5 catalyst. Appl. Catal., A 2010, 386, 109–115. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meler, W.M. Crystal Structure and Structural-Related Properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Ueno, K.; Negishi, H.; Okano, T.; Tawarayama, H.; Ishikawa, S.; Miyamoto, M.; Uemiya, S.; Ouni, Y. Effects of Silica-Particle Coating on Silica Support for the Fabrication of High-Performance Silicalite-1 Membranes by Gel-Free Steam-Assisted Conversion. Membranes 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Lin, Y.S.; Hu, M.Z.-C.; Peascoe, R.A.; Payzant, E.A. Template-removal-associated microstructural development of porous-ceramics-supported MFI zeolite membranes. Microporous Mesoporous Mater. 2000, 34, 241–253. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, H.-K.; Snyder, M.A.; Stoeger, J.A.; Masel, R.I.; Tsapatsis, M. Grain Boundary Defect Elimination in a Zeolite Membrane by Rapid Thermal Processing. Science 2009, 325, 590–593. [Google Scholar] [CrossRef]
- Akhtar, F.; Ojuva, A.; Wirawan, S.K.; Hedlund, J.; Bergstrom, L. Hierarchically porous binder-free silicalite-1 discs: A novel support for all-zeolite membranes. J. Mater. Chem. 2011, 21, 8822–8828. [Google Scholar] [CrossRef]
- Akhtar, F.; Sjoberg, E.; Korelskiy, D.; Rayson, M.; Hedlund, J.; Bergstrom, L. Preparation of graded silicalite-1 substrates for all-zeolite membranes with excellent CO2/H2 separation performance. J. Membr. Sci. 2015, 493, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Hedlund, J.; Jareman, F.; Bons, A.-J.; Anthonis, M. A masking technique for high quality MFI membranes. J. Membr. Sci. 2003, 222, 163–179. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, M.Y.; Stottrup, B.L.; Tsapatsis, M. para-Xylene Ultra-selective Zeolite MFI Membranes Fabricated from Nanosheet Monolayers at the Air–Water Interface. Angew. Chem. Int. Ed. 2018, 57, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Bronic, J.; Subotic, B.; Lv, X.-C.; Yang, Z.-J.; Tang, Y. Controllable and SDA-free synthesis of sub-micrometer sized zeolite ZSM-5. Part 1: Influence of alkalinity on the structural, particulate and chemical properties of the products. Microporous Mesoporous Mater. 2011, 139, 197–206. [Google Scholar] [CrossRef]
- Sakai, M.; Fujimaki, N.; Kobayashi, G.; Yasuda, N.; Oshima, Y.; Seshimo, M.; Matsukata, M. Formation process of *BEA-type zeolite membrane under OSDA-free conditions and its separation property. Microporous Mesoporous Mater. 2019, 284, 360–365. [Google Scholar] [CrossRef]
- Pan, M.; Lin, Y.S. Template-free secondary growth synthesis of MFI type zeolite membrane. Microporous Mesoporous Mater. 2001, 43, 319–327. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Curvature and Parametric Sensitivity in Models for Adsorption in Micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Argon porosimetry of selected molecular sieves: Experiments and examination of the adapted Horvath-Kawazoe model. Microporous Mater. 1995, 3, 531–542. [Google Scholar] [CrossRef]
- Li, G.; Kikuchi, E.; Matsukata, M. The control of phase and orientation in zeolite membranes by the secondary growth method. Microporous Mesoporous Mater. 2003, 62, 211–220. [Google Scholar] [CrossRef]
- Sommer, S.; Melin, T.; Falconer, J.L.; Noble, R.D. Transport of C6 isomers through ZSM-5 zeolite membranes. J. Membr. Sci. 2003, 224, 51–67. [Google Scholar] [CrossRef]
- Cavalcante, L., Jr.; Ruthven, D.M. Adsorption of Branched and Cyclic Paraffins in Silicalite. 2. Kinetics. Ind. Eng. Chem. Res. 1995, 34, 185–191. [Google Scholar] [CrossRef]
- Tsuru, T.; Hino, T.; Yoshioka, T.; Asaeda, M. Permporometry characterization of microporous ceramic membranes. J. Membr. Sci. 2001, 186, 257–265. [Google Scholar] [CrossRef]











| Support | Membrane | Feed Pressure/kPa | Temperature/K | p-Xylene Permeance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| p-Xylene | o-Xylene | m-Xylene | /10−8 mol m−2 s−1 Pa−1 | αp/o | αp/m | ||||
| α-Alumina Disc | Ultra-Thin MFI | 0.27 | 0.59 | − | 373 | 60 | 3.2 | − | [6] |
| Stainless steel tube | Ba-ZSM-5 | 0.23 | 0.26 | 0.83 | 423 | 7.0 | 13.5 | 8.2 | [8] |
| Stainless steel tube | Ba-ZSM-5 | 0.23 | 0.26 | 0.83 | 673 | 5.4 | 8.4 | 6.2 | [8] |
| α-Alumina disc | b-Oriented siliceous ZSM-5 | 0.45 | 0.35 | − | 373 | 20 | 600 | − | [9] |
| α-Alumina disc | b-Oriented siliceous ZSM-5 | 0.50 | 0.45 | − | 373 | 25 | 60 | − | [10] |
| α-Alumina tube | Composite MFI-alumina tube | 0.63 | 0.32 | 0.27 | 473 | 1.1 | >400 | − | [11] |
| Sintered silica fibre | b-Oriented MFI nanosheet | 0.30 | 0.31 | − | 323 | 0.85 | 32.3 | − | [19] |
| Sintered silica fibre | b-Oriented MFI nanosheet | 0.37 | 0.35 | − | 423 | 55.5 | 1989 | − | [19] |
| Sintered silica fibre | b-Oriented MFI nanosheet | 0.50 | 0.50 | − | 373 | 2.5 | ca. 3500 | − | [20] |
| Sintered silica fibre | b-Oriented MFI nanosheet | 0.50 | 0.50 | − | 423 | 29 | ca. 8000 | − | [20] |
| α-Alumina tube | (h0l)-Oriented silcialite-1 | 0.40 | 0.40 | 0.40 | 373 | 6.5 | >100 | >100 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, M.; Kaneko, T.; Sasaki, Y.; Sekigawa, M.; Matsukata, M. Formation Process of Columnar Grown (101)-Oriented Silicalite-1 Membrane and Its Separation Property for Xylene Isomer. Crystals 2020, 10, 949. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100949
Sakai M, Kaneko T, Sasaki Y, Sekigawa M, Matsukata M. Formation Process of Columnar Grown (101)-Oriented Silicalite-1 Membrane and Its Separation Property for Xylene Isomer. Crystals. 2020; 10(10):949. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100949
Chicago/Turabian StyleSakai, Motomu, Takuya Kaneko, Yukichi Sasaki, Miyuki Sekigawa, and Masahiko Matsukata. 2020. "Formation Process of Columnar Grown (101)-Oriented Silicalite-1 Membrane and Its Separation Property for Xylene Isomer" Crystals 10, no. 10: 949. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100949