Facile Synthesis, Characterization, Anti-Microbial and Anti-Oxidant Properties of Alkylamine Functionalized Dumb-Bell Shaped Copper-Silver Nanostructures
Abstract
:1. Introduction
2. Experimental Details
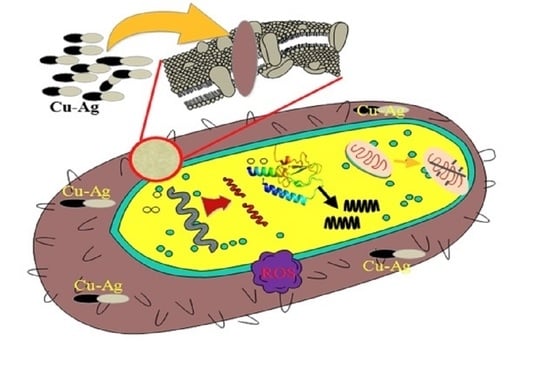
2.1. Antimicrobial Activities
2.2. Antioxidant Activity Studies
3. Results and Discussion
Antioxidant Activity of Cu-Ag Nanostructures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, S.; Tao, F.; Tang, Y.; Li, Y.; Yu, J. Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Zimmerman, J.B.; Plata, D.L.; Hutchison, J.E.; Anastas, P.T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015, 44, 5758–5777. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 12, 908–931. [Google Scholar] [CrossRef]
- Anandkumar, M.; Vinothkumar, G.; Babu, K.S. Synergistic effect of gold supported on redox active cerium oxide nanoparticles for the catalytic hydrogenation of 4-nitrophenol. New J. Chem. 2017, 41, 6720–6729. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef]
- Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2010, 26, 2885–2893. [Google Scholar] [CrossRef]
- Lv, J.-J.; Wang, A.-J.; Ma, X.; Xiang, R.-Y.; Chen, J.-R.; Feng, J.-J. One-pot synthesis of porous Pt–Au nanodendrites supported on reduced graphene oxide nanosheets toward catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2014, 3, 290–296. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Goodman, D.W. The nature of the metal-metal bond in bimetallic surfaces. Science 1992, 257, 897–903. [Google Scholar] [CrossRef]
- Francesco, I.N.; Fontaine-Vive, F.; Antoniotti, S. Synergy in the catalytic activity of bimetallic nanoparticles and new synthetic methods for the preparation of fine chemicals. ChemCatChem 2014, 6, 2784–2791. [Google Scholar] [CrossRef]
- Fu, F.; Cheng, Z.; Lu, J. Synthesis and use of bimetals and bimetal oxides in contaminants removal from water: A review. RSC Adv. 2015, 5, 85395–85409. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Zang, W.; Li, G.; Wang, L.; Zhang, X. Catalytic hydrogenation by noble-metal nanocrystals with well-defined facets: A review. Catal. Sci. Technol. 2015, 5, 2532–2553. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Wu, W.; Lei, M.; Yang, S.; Zhou, L.; Liu, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. A one-pot route to the synthesis of alloyed Cu/Ag bimetallic nanoparticles with different mass ratios for catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 3450–3455. [Google Scholar] [CrossRef]
- Peng, X.; Pan, Q.; Rempel, G.L. Bimetallic dendrimer-encapsulated nanoparticles as catalysts: A review of the research advances. Chem. Soc. Rev. 2008, 37, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Hoon Byeon, J.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu monometallic and Ag/Cu bimetallic nanoparticle—Graphene composites with enhanced antibacterial performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, J.; Osaka, T.; Park, S. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.M.; Tahziba, A.; Bayati, K.; Pouladzadeh, M.; Zohuriaan, M.J. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. Eur. Polym. J. 2014, 40, 1355–1361. [Google Scholar] [CrossRef]
- Bansal, A.; Verma, S.S. Optical response of noble metal alloy. Phys. Lett. A 2015, 379, 163–169. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Sindhe, M.A.; Satyanarayan, N.D.; Pramod, S.N.; Nagaraja, K. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 108–115. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J. Biological evaluation of silver nanoparticles obtained from T. arjuna bark extract as both reducing and capping agent. J. Clust. Sci. 2014, 25, 1449–1462. [Google Scholar] [CrossRef]
- Banik, M.; Patra, M.; Dutta, D.; Mukherjee, R.; Basu, T. A simple robust method of synthesis of copper–silver core–shell nano-particle: Evaluation of its structural and chemical properties with anticancer potency. Nanotechnology 2018, 29, 325102. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Nath, S.; Praharaj, S.; Panigrahi, S.; Kundu, S.; Ghosh, S.K.; Basu, S.; Pal, T. Hexadecylamine capped silver organosol: A substrate for surface enhanced Raman scattering. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 145–149. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Fang, Y.; Chen, S.; Li, N.; Zhou, Q. 1-Hexadecylamine as both reducing agent and stabilizer to synthesize Au and Ag nanoparticles and their SERS application. J. Nanopart. Res. 2011, 13, 1929–1936. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Kim, H. Synthesis of shape and size-dependent CuAg bimetallic dumbbell structures for organic pollutant hydrogenation. Physica. E Low-Dimens. Syst. Nanostruct. 2018, 102, 44–49. [Google Scholar] [CrossRef]
- Bastos, C.A.P.; Faria, N.; Wills, J.; Malmberg, P.; Scheers, N.; Rees, P.; Powell, J.J. Copper nanoparticles have negligible direct antibacterial impact. NanoImpact 2020, 17, 100192. [Google Scholar] [CrossRef]
- Lin, Y.S.E.; Vidic, R.D.; Stout, J.E.; McCartney, C.A.; Yu, V.L. Inactivation of Mycobacterium avium by copper and silver ions. Water Res. 1998, 32, 1997–2000. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chou, C.-C. Antioxidative activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 2004, 37, 883–889. [Google Scholar] [CrossRef]
- Srihasam, S.; Thyagarajan, K.; Korivi, M.; Lebaka, V.R.; Mallem, S.P.R. Phytogenic generation of NiO nanoparticles using stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, E.; Asti, M.; Benassi, R.; Pignedoli, F.; Saladini, M. Metal binding ability of curcumin derivatives: A theoretical vs. experimental approach. Dalton Trans. 2013, 42, 5304–5313. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]







| Activity Name | Zone of Inhibition (mm) | MIC (µg/mL) | MBC (µg/mL) | ||
|---|---|---|---|---|---|
| P | Cu-Ag (50 µg/mL) | Cu-Ag (100 µg/mL) | |||
| E. coli | 23 | 20 | 28 | 23 | 85 |
| K. pneumoniae | 15 | 15 | 20 | 36 | 97 |
| B. subtilis | 18 | 14 | 20 | 38 | 132 |
| S. aureus | 20 | 12 | 16 | 40 | 148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallikarjuna, K.; Al-Mohaimeed, A.M.; Al-Farraj, D.A.; Reddy, L.V.; Vasudeva Reddy, M.R.; Mohammed, A. Facile Synthesis, Characterization, Anti-Microbial and Anti-Oxidant Properties of Alkylamine Functionalized Dumb-Bell Shaped Copper-Silver Nanostructures. Crystals 2020, 10, 966. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10110966
Mallikarjuna K, Al-Mohaimeed AM, Al-Farraj DA, Reddy LV, Vasudeva Reddy MR, Mohammed A. Facile Synthesis, Characterization, Anti-Microbial and Anti-Oxidant Properties of Alkylamine Functionalized Dumb-Bell Shaped Copper-Silver Nanostructures. Crystals. 2020; 10(11):966. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10110966
Chicago/Turabian StyleMallikarjuna, Koduru, Amal M. Al-Mohaimeed, Dunia A. Al-Farraj, Lebaka Veeranjaneya Reddy, Minnam Reddy Vasudeva Reddy, and Arifullah Mohammed. 2020. "Facile Synthesis, Characterization, Anti-Microbial and Anti-Oxidant Properties of Alkylamine Functionalized Dumb-Bell Shaped Copper-Silver Nanostructures" Crystals 10, no. 11: 966. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10110966