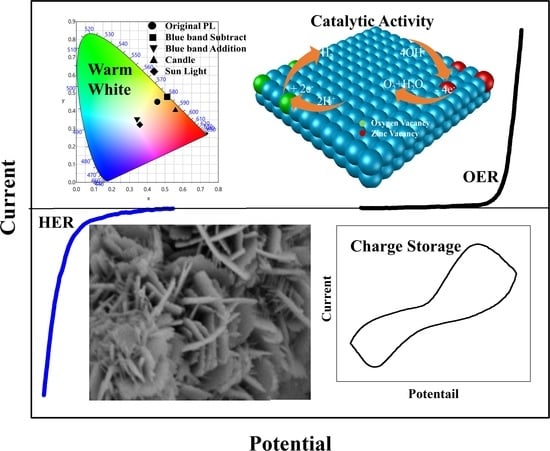
Multifunctional Hierarchically Architectured ZnO for Luminescence, Photocatalytic, Electrocatalytic, and Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Structural Assessment
3.2. Positron Annihilation Spectroscopy
3.3. Luminescence
3.4. White Light Photocatalytic Activity
3.5. Energy Storage
3.6. Electrocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, W.L.; Zheng, M.J.; Ding, G.Q.; Shen, W.Z. Fabrication and optical properties of highly ordered ZnO nanodot arrays. Chem. Phys. Lett. 2005, 411, 37–42. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nano lasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ji, Y.L.; Xu, H.; Simon, P.; Wu, Z. Regularly shaped, single-crystalline ZnO nanorods with wurtzite structure. J. Am. Chem. Soc. 2002, 124, 14864–14865. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.Y.; Ding, Y.; Yang, R.; Wang, Z.L. Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science 2004, 303, 1348–1351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun, L.; Liao, C.; Yan, C. A simple route towards tubular ZnO. Chem. Commun. 2002, 3, 262–263. [Google Scholar] [CrossRef]
- Fan, H.J.; Scholz, R.; Kolb, F.M.; Zacharias, M.; Gösele, U. Growth mechanism and characterization of zinc oxide microcages. Solid State Commun. 2004, 130, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Lao, J.Y.; Wen, J.G.; Ren, Z.F. Hierarchical ZnO nanostructures. Nano Lett. 2002, 2, 1287–1291. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kim, H.W. Synthesis of ZnO nanorod on bare Si substrate using metal organic chemical vapor deposition. Phys. B Condens. Matter 2003, 328, 368–371. [Google Scholar] [CrossRef]
- Ng, H.T.; Chen, B.; Li, J.; Han, J.; Meyyappan, M.; Wu, J.; Li, S.X.; Haller, E.E. Optical properties of single-crystalline ZnO nanowires on m-sapphire. Appl. Phys. Lett. 2003, 82, 2023–2025. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Ruh, H.; Lee, H.-J.; Shim, H.-W.; Suh, E.-K.; Lee, C.J. Low temperature growth and photoluminescence of well-aligned zinc oxide nanowires. Chem. Phys. Lett. 2002, 363, 134–138. [Google Scholar] [CrossRef]
- Gao, P.X.; Wang, Z.L. Substrate atomic-termination-induced anisotropic growth of ZnO nanowires/nanorods by the VLS process. J. Phys. Chem. B 2004, 108, 7534–7537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, H.; Luo, X.; Chen, X.; Yu, D.; Wang, R. Synthesis, microstructure, and growth mechanism of dendrite ZnO nanowires. J. Phys. Chem. B 2003, 107, 8289–8293. [Google Scholar] [CrossRef]
- Roy, V.A.L.; Djurišić, A.B.; Chan, W.K.; Gao, J.; Lui, H.F.; Surya, C. Luminescent and structural properties of ZnO nanorods prepared under different conditions. Appl. Phys. Lett. 2003, 83, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Jiansheng, J.; Wang, G.; Wang, Q.; Chen, Y.; Han, X.; Wang, X.; Hou, J.G. Synthesis and Characterization of Aligned ZnO Nanorods on Porous Aluminum Oxide Template. J. Phys. Chem. B 2004, 108, 11976. [Google Scholar]
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Li, Y.; Meng, G.W.; Zhang, L.D.; Phillipp, F. Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl. Phys. Lett. 2000, 76, 2011–2013. [Google Scholar] [CrossRef]
- Zheng, M.J.; Zhang, L.D.; Li, G.H.; Shen, W.Z. Fabrication and optical properties of large-scale uniform zinc oxide nanowire arrays by one-step electrochemical deposition technique. Chem. Phys. Lett. 2002, 363, 123–128. [Google Scholar] [CrossRef]
- Jia, W.; Dang, S.; Liu, H.; Zhang, Z.; Yu, C.; Liu, X.; Xu, B. Evidence of the formation mechanism of ZnO in aqueous solution. Mater. Lett. 2012, 82, 99–101. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Sun, X.; Li, H. Preparation of nanocrystalline metal oxide powders with the surfactant-mediated method. Inorg. Chem. Commun. 2002, 5, 751. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Liu, H.; Wang, X. Growth of well-defined ZnO microparticles with additives from aqueous solution. J. Solid State Chem. 2005, 178, 855–860. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Li, D.; Ma, X.; Li, S.; Que, D. Controllable growth of ZnO microcrystals by a capping-molecule-assisted hydrothermal process. Cryst. Growth Des. 2005, 5, 547–550. [Google Scholar] [CrossRef]
- Govender, K.; Boyle, D.S.; Kenway, P.B.; O’Brien, P. Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J. Mater. Chem. 2004, 14, 2575–2591. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, S.; Rajendar, V.; Park, S.H. Fabrication of ZnO Thin Films by Sol-Gel Spin Coating and Their UV and White-Light Emission Properties. J. Electron. Mater. 2017, 46, 6029–6037. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Seong, K.S.; Park, S.H. Single-Crystalline ZnO/Graphene Quantum Dots Phosphors-Converted White Light-Emitting Diodes. IEEE Photonics Technol. Lett. 2019, 31, 203–205. [Google Scholar] [CrossRef]
- Ubaidullah, M.; Al-Enizi, A.M.; Shaikh, S.; Ghanem, M.A.; Mane, R.S. Waste PET plastic derived ZnO@NMC nanocomposite via MOF-5 construction for hydrogen and oxygen evolution reactions. J. King Saud Univ. Sci. 2020, 32, 2397–2405. [Google Scholar] [CrossRef]
- Wang, N.; Tao, B.; Miao, F.; Zang, Y. Electrodeposited Pd/graphene/ZnO/nickel foam electrode for the hydrogen evolution reaction. RSC Adv. 2019, 9, 33814–33822. [Google Scholar] [CrossRef] [Green Version]
- Aryanto, D.; Hastuti, E.; Taspika, M.; Anam, K.; Isnaeni, I.; Widayatno, W.B.; Wismogroho, A.S.; Marwoto, P.; Nuryadin, B.W.; Noviyanto, A. Characteristics and photocatalytic activity of highly c-axis-oriented ZnO thin films. J. Sol-Gel Sci. Technol. 2020, 96, 226–235. [Google Scholar] [CrossRef]
- Xiao, X.; Han, B.; Chen, G.; Wang, L.; Wang, Y. Preparation and electrochemical performances of carbon sphere @ZnO core-shell nanocomposites for supercapacitor applications. Sci. Rep. 2017, 7, 40167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, B.K. Carbon nanotube-ZnO nanocomposite electrodes for supercapacitors. Solid State Ion. 2009, 180, 1525–1528. [Google Scholar] [CrossRef]
- Lu, T.; Pan, L.; Li, H.; Zhu, G.; Lv, T.; Liu, X.; Sun, Z.; Chen, T.; Chua, D.H. Microwave-assisted synthesis of graphene-ZnO nanocomposite for electrochemical supercapacitors. J. Alloys Compond. 2011, 509, 5488–5492. [Google Scholar] [CrossRef]
- Ayaz, S.; Mishra, P.; Sen, S. Structure correlated optoelectronic and electrochemical properties of Al/Li modified ZnO. J. Appl. Phys. 2019, 126, 024302. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Pedersen, N.J.; Eldrup, M. PATFIT-88: A Data-processing System for Positron Annihilation Spectra on Mainframe and Personal Computers; RISØ-M-2740; Risø National Laboratory: Roskilde, Denmark, 1989; Available online: www.risoe.dk/rispubl/reports/ris-m-2740.pdf (accessed on 21 September 2020).
- Musić, S.; Dragčević, Đ.; Popović, S. Influence of synthesis route on the formation of ZnO particles and their morphologies. J. Alloys Compd. 2007, 429, 242–249. [Google Scholar] [CrossRef]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Lippincott, E.R.; Psellos, J.A.; Tobin, M.C. The Raman spectra and structures of aluminate and zincate ions. J. Chem. Phys. 1952, 20, 536. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibanez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Look, D.C.; Reynolds, D.C.; Sizelove, J.R.; Jones, R.L.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Electrical properties of bulk ZnO. Solid State Commun. 1998, 105, 399–401. [Google Scholar] [CrossRef]
- Tuomisto, F.; Ranki, V.; Saarinen, K.; Look, D.C. Evidence of the Zn vacancy acting as the dominant acceptor in n-type ZnO. Phys. Rev. Lett. 2003, 91, 205502. [Google Scholar] [CrossRef] [Green Version]
- Brauer, G.; Anwand, W.; Grambole, D.; Grenzer, J.; Skorupa, W.; Čížek, J.; Kuriplach, J.; Procházka, I.; Ling, C.C.; So, C.K. Identification of Zn-vacancy-hydrogen complexes in ZnO single crystals: A challenge to positron annihilation spectroscopy. Phys. Rev. B 2009, 79, 115212. [Google Scholar] [CrossRef] [Green Version]
- Anwand, W.; Brauer, G.; Cowan, T.E.; Grambole, D.; Skorupa, W.; Čížek, J.; Kuriplach, J.; Procházka, I.; Egger, W.; Sperr, P. Structural characterization of H plasma-doped ZnO single crystals by positron annihilation spectroscopies. Phys. Status Solidi A 2010, 207, 2415–2425. [Google Scholar] [CrossRef]
- Xie, R.; Sekiguchi, T.; Ishigaki, T.; Ohashi, N.; Li, D.; Yang, D.; Liu, B.; Bando, Y. Enhancement and patterning of ultraviolet emission in ZnO with an electron beam. Appl. Phys. Lett. 2006, 88, 134103. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Maekawa, M.; Yamamoto, S.; Kawasuso, A.; Yuan, X.L.; Sekiguchi, T.; Suzuki, R.; Ohdaira, T. Evolution of voids in Al+-implanted ZnO probed by a slow positron beam. Phys. Rev. B 2004, 69, 035210. [Google Scholar] [CrossRef] [Green Version]











| ZnO at Different pH | Positron Lifetime (ps) | Defect Relative Intensities | |||||
|---|---|---|---|---|---|---|---|
| τ1 | τ2 | τ3 | I1 | I2 | I3 | ||
| 9 | Raw | 166.1 ± 11.1 | 317.6 ± 23.7 | 812.7 ± 226.2 | 50.4 ± 8.5 | 48.03 ± 7.4 | 1.57 ± 1.4 |
| 250 | 183.7 ± 4.1 | 400 ± 7.6 | 3168.2 ± 249.1 | 39.11 ± 2.1 | 40.36 ± 2.1 | 0.54 ± 0.1 | |
| 11.5 | Raw | 168.0 ± 5.6 | 340.0 ± 11.6 | 1360.5 ± 248.3 | 55.74 ± 3.9 | 43.57 ± 3.7 | 0.69 ± 0.3 |
| 250 | 197.5 ± 3.2 | 424 ± 9.4 | 4238.8 ± 155.2 | 67.46 ± 1.9 | 31.36 ± 1.9 | 1.18 ± 0.1 | |
| 13 | Raw | 198.3 ± 2.3 | 507.9 ± 15.7 | 2161.2 ± 97.6 | 76.05 ± 1.2 | 22.01 ± 1.4 | 1.94 ± 0.2 |
| 250 | 185.6 ± 3.9 | 393.3 ± 7.4 | 3936.6 ± 131.7 | 58.10 ± 2.2 | 40.58 ± 2.2 | 1.31 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.P.; Kumar, M.; Reddy, B.P.; Sunny; Gangwar, R.K.; Rath, C. Multifunctional Hierarchically Architectured ZnO for Luminescence, Photocatalytic, Electrocatalytic, and Energy Storage Applications. Crystals 2020, 10, 1025. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111025
Singh VP, Kumar M, Reddy BP, Sunny, Gangwar RK, Rath C. Multifunctional Hierarchically Architectured ZnO for Luminescence, Photocatalytic, Electrocatalytic, and Energy Storage Applications. Crystals. 2020; 10(11):1025. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111025
Chicago/Turabian StyleSingh, V. P., Mirgender Kumar, B. Purusottam Reddy, Sunny, R. K. Gangwar, and Chandana Rath. 2020. "Multifunctional Hierarchically Architectured ZnO for Luminescence, Photocatalytic, Electrocatalytic, and Energy Storage Applications" Crystals 10, no. 11: 1025. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111025