Protocol Comparison for Organic Residue Analyses from Waterproofing Materials and Shards of Roman Archaeological Amphorae
Abstract
:1. Introduction
2. Materials
2.1. Archaeological Samples
2.2. Solvents and Reagents
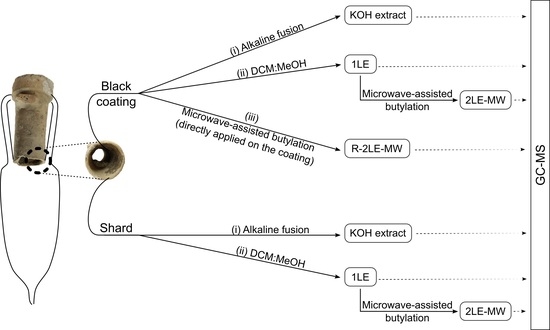
3. Methods
3.1. Optimization of the Acid-Catalyzed Esterification: Microwave-Assisted-Butylation
3.2. Analytical Procedures for Inorganic Shards
3.3. Analytical Procedures for Organic Coatings
3.4. GC-MS Analyses
3.5. Radar Plot Construction
4. Results and Discussion
4.1. Optimization of the Acid-Catalyzed Butylation
4.2. Extracting Capacities Comparison on Archaeological Shards
4.3. Extracting Capacities Comparison on Archaeological Coatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBT | Dibutyl tartrate |
| DCM | Dichloromethane |
| DHA | Dehydroabietic acid |
| DHAM | Dehydroabietic methyl ester |
| DiOH-DHA | 7,15-dihydroxy-dehydroabietic acid |
| DiOH-DHAM | 7,15-dihydroxy-dehydroabietic methyl ester |
| KOH | Potassium hydroxide |
| OH-DHA | Hydroxy-dehydroabietic acid (3-hydroxy-dehydroabietic acid; 7-hydroxy-dehydroabietic acid; 15-hydroxy-dehydroabietic acid) |
| OH-DHAM | Hydroxy-dehydroabietic methyl ester (3-hydroxy-dehydroabietic methyl ester; 7-hydroxy-dehydroabietic methyl ester; 15-hydroxy-dehydroabietic methyl ester) |
| Oxo-DHA | 7-oxo-dehydroabietic acid |
| Oxo-DHAM | 7-oxo-dehydroabietic methyl ester |
| Oxo-OH-DHA | 7-oxo-15-hydroxy-dehydroabietic acid |
| Oxo-OH-DHAM | 7-oxo-15-hydroxy-dehydroabietic methyl ester |
References
- Heron, C.; Evershed, R.P. The analysis of organic residues and the study of pottery use. Archaeol. Method Theory 1993, 5, 247–284. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, P.E.; Michel, R.H. The analytical and archaeological challenge of detecting ancient wine: Two case studies from the Ancient Near East. In The Origins and Ancient History of Wine; Routledge: Langhorne, PA, USA, 1996; pp. 57–65. [Google Scholar]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.-s.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, R.H.; McGovern, P.E.; Badler, V.R. The first wine & beer. Anal. Chem. 1993, 65, 408A–413A. [Google Scholar] [CrossRef]
- Garnier, N.; Richardin, P.; Cheynier, V.; Regert, M. Characterization of thermally assisted hydrolysis and methylation products of polyphenols from modern and archaeological vine derivatives using gas chromatography–mass spectrometry. Anal. Chim. Acta 2003, 493, 137–157. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Gugić, M.; Roje, M. Chemical profile of the organic residue from ancient amphora found in the adriatic sea determined by direct GC and GC-MS analysis. Molecules 2011, 16, 7936–7948. [Google Scholar] [CrossRef]
- Pecci, A.; Giorgi, G.; Salvini, L.; Cau Ontiveros, M.Á. Identifying wine markers in ceramics and plasters using gas chromatography–mass spectrometry. Experimental and archaeological materials. J. Archaeol. Sci. 2013, 40, 109–115. [Google Scholar] [CrossRef]
- Stern, B.; Heron, C.; Tellefsen, T.; Serpico, M. New investigations into the Uluburun resin cargo. J. Archaeol. Sci. 2008, 35, 2188–2203. [Google Scholar] [CrossRef]
- Drieu, L.; Rageot, M.; Wales, N.; Stern, B.; Lundy, J.; Zerrer, M.; Gaffney, I.; Bondetti, M.; Spiteri, C.; Thomas-Oates, J.; et al. Is it possible to identify ancient wine production using biomolecular approaches? STAR Sci. Technol. Archaeol. Res. 2020, 6, 16–29. [Google Scholar] [CrossRef]
- Garnier, N.; Valamoti, S.M. Prehistoric wine-making at Dikili Tash (Northern Greece): Integrating residue analysis and archaeobotany. J. Archaeol. Sci. 2016, 74, 195–206. [Google Scholar] [CrossRef]
- Pecci, A.; Borgna, E.; Mileto, S.; Dalla Longa, E.; Bosi, G.; Florenzano, A.; Mercuri, A.M.; Corazza, S.; Marchesini, M.; Vidale, M. Wine consumption in Bronze Age Italy: Combining organic residue analysis, botanical data and ceramic variability. J. Archaeol. Sci. 2020, 123, 105256. [Google Scholar] [CrossRef]
- Fujii, H.; Mazzitelli, J.B.; Adilbekov, D.; Olmer, F.; Mathe, C.; Vieillescazes, C. FT-IR and GC–MS analyses of Dressel IA amphorae from the Grand Congloué 2 wreck. J. Archaeol. Sci. Rep. 2019, 28, 102007. [Google Scholar] [CrossRef]
- Fujii, H.; Krausz, S.; Olmer, F.; Mathe, C.; Vieillescazes, C. Analysis of organic residues from the Châteaumeillant oppidum (Cher, France) using GC–MS. J. Cult. Herit. 2021, 51, 50–58. [Google Scholar] [CrossRef]
- Guasch-Jané, M.R.; Ibern-Gómez, M.; Andrés-Lacueva, C.; Jáuregui, O.; Lamuela-Raventós, R.M. Liquid chromatography with mass spectrometry in tandem mode applied for the identification of wine markers in residues from ancient Egyptian vessels. Anal. Chem. 2004, 76, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Jané, M.R.; Andrés-Lacueva, C.; Járegui, O.; Lamuela-Raventós, R.M. The origin of the ancient Egyptian drink Shedeh revealed using LC/MS/MS. J. Archaeol. Sci. 2006, 33, 98–101. [Google Scholar] [CrossRef]
- Barnard, H.; Dooley, A.N.; Areshian, G.; Gasparyan, B.; Faull, K.F. Chemical evidence for wine production around 4000 BCE in the Late Chalcolithic Near Eastern highlands. J. Archaeol. Sci. 2011, 38, 977–984. [Google Scholar] [CrossRef]
- Manzano, E.; Cantarero, S.; García, A.; Adroher, A.; Vílchez, J.L. A multi-analytical approach applied to the archaeological residues in Iberian glasses. Earliest evidences on the consumption of fermented beverages in votive rituals. Microchem. J. 2016, 129, 286–292. [Google Scholar] [CrossRef]
- Rageot, M.; Mötsch, A.; Schorer, B.; Bardel, D.; Winkler, A.; Sacchetti, F.; Chaume, B.; Della Casa, P.; Buckley, S.; Cafisso, S.; et al. New insights into Early Celtic consumption practices: Organic residue analyses of local and imported pottery from Vix-Mont Lassois. PLoS ONE 2019, 14, e0218001. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarejo, P.; Hernandez, F. Organic acids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef] [Green Version]
- Pecci, A.; Domínguez-Bella, S.; Buonincontri, M.P.; Miriello, D.; De Luca, R.; Di Pasquale, G.; Cottica, D.; Bernal Casasola, D. Combining residue analysis of floors and ceramics for the study of activity areas at the Garum Shop at Pompeii. Archaeol. Anthropol. Sci. 2018, 10, 485–502. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine Stabilization and Treatment, Handbook of Enology; Johan Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Blanco-Zubiaguirre, L.; Olivares, M.; Castro, K.; Carrero, J.A.; García-Benito, C.; García-Serrano, J.Á.; Pérez-Pérez, J.; Pérez-Arantegui, J. Wine markers in archeological potteries: Detection by GC-MS at ultratrace levels. Anal. Bioanal. Chem. 2019, 411, 6711–6722. [Google Scholar] [CrossRef]
- Correa-Ascencio, M.; Evershed, R.P. High throughput screening of organic residues in archaeological potsherds using direct acidified methanol extraction. Anal. Methods 2014, 6, 1330. [Google Scholar] [CrossRef]
- Pecci, A.; Clarke, J.; Thomas, M.; Muslin, J.; van der Graaff, I.; Toniolo, L.; Miriello, D.; Crisci, G.; Buonincontri, M.; Di Pasquale, G. Use and reuse of amphorae. Wine residues in Dressel 2–4 amphorae from Oplontis Villa B (Torre Annunziata, Italy). J. Archaeol. Sci. Rep. 2017, 12, 515–521. [Google Scholar] [CrossRef]
- Drieu, L.; Orecchioni, P.; Capelli, C.; Meo, A.; Lundy, J.; Sacco, V.; Arcifa, L.; Molinari, A.; Carver, M.; Craig, O.E. Chemical evidence for the persistence of wine production and trade in Early Medieval Islamic Sicily. Proc. Natl. Acad. Sci. USA 2021, 118, e2017983118. [Google Scholar] [CrossRef] [PubMed]
- Frère, D.; Garnier, N. Dairy Product and Wine in Funerary Rituals: The Case of a Hellenistic Etruscan Tomb. J. Hist. Archaeol. Anthropol. Sci. 2017, 1, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Tchernia, A. Premiers Résultats Des Fouilles de Juin 1968 Sur L’épave 3 du Planier; Etudes Classiques: Bouches-du-Rhône, France, 1968; Volume III. [Google Scholar]
- Tchernia, A. Les fouilles sous-marines de Planier (Bouches-du-Rhône). C. R. Séances L’académie Inscr. B-Lett. 1969, 113, 292–309. [Google Scholar] [CrossRef]
- Olmer, F. Les Amphores Sont-Elles Utiles à la Chronologie de la fin de L’âge du Fer Annexe. Philippe Barral. Regards sur la Chronologie de la fin de l’âge du Fer (IIIe-Ier siècle avant J.-C.) en Gaule non Méditerranéenne. Actes de la Table Ronde Tenue à Bibracte “Chronologie de la fin de l’âge du Fer (IIIe-Ier siècle avant J.-C.) Dans l’est de la France et les Régions Voisines ”, 22, Bibracte. 2012. ISBN 978-2-909668-74-1. Available online: https://halshs.archives-ouvertes.fr/halshs-01328970/document (accessed on 20 September 2021).
- Cipriano, M.T.; Carre, M.-B. Production et typologie des amphores sur la côte adriatique de l’Italie. Amphores romaines et histoire economique. Dix ans de recherche. Actes du colloque de Sienne (22–24 mai 1986). Publ. L’école Française Rome 1989, 114, 67–104. [Google Scholar]
- Panella, C. Anfore e archeologia subacquea. In Archeologia Subacquea. Come Opera L’archeologo Sott’acqua. Storie Dalle Acque; All’insegna del Giglio: Firenze, Italy, 1998; pp. 531–559. [Google Scholar]
- Lamboglia, N. La Nave Romana di Albenga. Rev. Degli Stud. Liguri 1952, 18, 131–236. [Google Scholar]
- Okan, E.; Atila, C.; Akyol, A.A. The production of chios-style amphorae at a ceramic workshop in Phocaea (Foça). Mediterr. Archaeol. Archaeom. 2015, 15, 259–276. [Google Scholar] [CrossRef]
- Bonifay, M. Amphores de l’Afrique romaine: Nouvelles avancées sur la production, la typo-chronologie et le contenu. In Amphorae ex Hispania. Paisajes de producción y consumo, III Congreso internacional de la SECAH-EX OFFICINA HISPANA (Tarragone, 10-13 décembre 2014); Monografías Ex Officina Hispana III: Tarragona, Spain, 2016; pp. 595–611. [Google Scholar]
- Peña, J.T. Roman Pottery in the Archaeological Record; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780511499685. [Google Scholar]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9783527314522. [Google Scholar]
- Garnier, N.; Bernal Casasola, D.; Driard, C.; Pinto, I.V. Looking for ancient fish products through invisible biomolecular residues in the Roman production vats from the Atlantic coast. J. Marit. Archaeol. 2018, 13, 285–328. [Google Scholar] [CrossRef]
- Mezzatesta, E.; Perraud, A.; Vieillescazes, C.; Mathe, C. GC–MS and PCA analyses of diterpenoids degradation state in 21 human mummies of Ancient Egypt dating from New Kingdom to Graeco-Roman Period. J. Cult. Herit. 2021, 47, 43–49. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; Colombini, M.P., Modugno, F., Eds.; John Wiley & Sons: Chichester, UK, 2009; ISBN 9780470741917. [Google Scholar]
- Modugno, F.; Ribechini, E.; Colombini, M.P. Chemical study of triterpenoid resinous materials in archaeological findings by means of direct exposure electron ionisation mass spectrometry and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1787–1800. [Google Scholar] [CrossRef]
- Proestos, C.; Bakogiannis, A.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Komaitis, M. High performance liquid chromatography analysis of phenolic substances in Greek wines. Food Control 2005, 16, 319–323. [Google Scholar] [CrossRef]
- Kawamura, K. Identification of C2-C10 ω-oxocarboxylic acids, pyruvic acid, and C2-C3 α-dicarbonyls in wet precipitation and aerosol samples by capillary GC and GC/MS. Anal. Chem. 1993, 65, 3505–3511. [Google Scholar] [CrossRef]
- Delmond, B.; Taran, M.; Valade, J. Réarrangements de squelette au cours de l’isomérisation catalysée par l’éthérate de trifluorure de bore d’epoxyde diterpénique. Tetrahedron Lett. 1978, 19, 4791–4794. [Google Scholar] [CrossRef]
- Taran, M.; Delmond, B. Part. IV—Obtention de nouveaux squelettes diterpeniques tetracycliques lors d’isomerisation d’epoxydes-8,9 diterpeniques. Tetrahedron 1986, 42, 4795–4806. [Google Scholar] [CrossRef]
- Fujita, E.; Fuji, K.; Nagao, Y.; Node, M. The Chemistry on Diterpenoids in 1978. Part-III. Bull. Inst. Chem. Res. Kyoto Univ. 1979, 57, 385–410. [Google Scholar]



| Amphora | Archaeological Site | Typology | Coating | Shard | Grape Derivatives | Pinaceae Products |
|---|---|---|---|---|---|---|
| 1014 | Planier 3 | Dressel 1 | X | X | Fermented | Wood tar |
| 749 | Planier 3 | Lamboglia 2 | X | X | Fermented | Wood tar |
| 6570a | Planier 3 | Dressel 1 | X | Fermented | Wood tar | |
| SFC1 | San Felice Circeo | Dressel 1 | X | Fermented | Wood tar | |
| SFC2 | San Felice Circeo | Dressel 1 | X | Fermented | Wood tar | |
| SFC3 | San Felice Circeo | Mañà C2 | X | Fermented | Wood tar | |
| SFC4 | San Felice Circeo | Greek-Italian | X | Fermented | Wood tar | |
| SFC5 | San Felice Circeo | Lamboglia 2 | X | Fermented | Wood tar | |
| 6904 | Planier 3 | Chios amphora | X | Fermented | Pitch | |
| 6828a | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar | |
| 6828a | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar | |
| 6565 | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar | |
| 6793 | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar | |
| 6545 | Planier 3 | Dressel 5 | X | Fermented | Wood tar | |
| 6566 | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar | |
| 6828c | Planier 3 | Lamboglia 2 | X | Fermented | Wood tar |
| Amphora | Protocol | Maleic ac. | Succinic ac. | Pyruvic ac. | Fumaric ac. | Malic ac. | Tartaric ac. | Syringic ac. | Retene | DHA | OH-DHA | Oxo-DHA | DiOH-DHA | Oxo-OH-DHA | DHAM | OH-DHAM | Oxo-DHAM | DiOH-DHAM | Oxo-OH-DHAM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1014 | KOH ext | - | + | - | + | - | - | + | + | + | +++ | + | - | - | + | + | - | - | + |
| DCM:MeOH | - | + | + | - | - | - | - | + | + | +++ | + | + | - | + | ++ | + | + | - | |
| 2LE-MW | + | + | - | + | + | + | + | ||||||||||||
| 749 | KOH ext | - | + | - | + | + | - | + | + | + | +++ | + | + | + | + | +++ | + | + | + |
| DCM:MeOH | - | + | + | - | - | - | - | + | + | +++ | + | + | + | + | +++ | + | + | + | |
| 2LE-MW | + | + | - | - | + | + | + | ||||||||||||
| 6904 | KOH ext | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| DCM:MeOH | - | + | + | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | |
| 2LE-MW | + | + | - | + | + | + | - | ||||||||||||
| 6828A | KOH ext | - | + | - | + | + | - | + | + | + | +++ | + | + | + | + | ++ | - | + | + |
| DCM:MeOH | - | + | + | + | - | - | - | + | + | +++ | - | + | + | + | +++ | + | + | + | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| 6828B | KOH ext | - | + | + | + | + | - | + | + | + | +++ | + | + | + | + | + | - | + | + |
| DCM:MeOH | - | + | + | - | - | - | + | + | + | ++ | - | - | - | + | +++ | + | - | - | |
| 2LE-MW | + | + | - | - | + | + | + | ||||||||||||
| 6565 | KOH ext | - | + | + | - | + | - | + | + | + | - | - | - | - | + | + | + | - | + |
| DCM:MeOH | - | + | + | - | - | - | - | + | + | ++ | - | - | - | + | ++ | + | - | + | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| 6793 | KOH ext | - | + | - | - | - | - | + | + | + | +++ | - | + | - | + | ++ | + | + | + |
| DCM:MeOH | - | + | + | - | - | - | + | + | + | + | - | - | - | + | - | - | - | - | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| 6545 | KOH ext | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - |
| DCM:MeOH | - | + | - | - | - | - | - | - | - | - | - | + | - | + | - | - | + | - | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| 6566 | KOH ext | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - |
| DCM:MeOH | - | - | + | - | - | - | - | + | + | - | - | + | - | + | + | - | + | - | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| 6828C | KOH ext | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - |
| DCM:MeOH | - | - | + | - | - | - | - | + | + | - | - | + | - | + | + | + | + | - | |
| 2LE-MW | - | + | - | - | + | + | + | ||||||||||||
| Amphora | Protocol | Maleic ac. | Succinic ac. | Pyruvic ac. | Fumaric ac. | Malic ac. | Tartaric ac. | Syringic ac. | Retene | DHA | OH-DHA | Oxo-DHA | DiOH-DHA | Oxo-OH-DHA | DHAM | OH-DHAM | Oxo-DHAM | DiOH-DHAM | Oxo-OH-DHAM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1014 | KOH ext | - | + | - | - | - | - | + | + | + | +++ | + | + | + | + | +++ | - | + | + |
| DCM:MeOH | - | + | - | + | - | - | + | + | + | ++ | - | + | - | + | +++ | + | + | + | |
| 2LE-MW | - | - | - | - | - | - | + | ||||||||||||
| R-2LE-MW | - | + | + | - | + | + | + | ||||||||||||
| 749 | KOH ext | - | + | - | + | + | - | + | + | + | +++ | + | + | + | + | ++ | - | + | + |
| DCM:MeOH | - | + | - | + | + | - | + | + | + | ++ | - | - | - | + | +++ | + | - | + | |
| 2LE-MW | - | - | + | - | + | + | - | ||||||||||||
| R-2LE-MW | - | + | + | - | + | + | + | ||||||||||||
| 6570a | KOH ext | - | + | - | + | + | - | + | + | + | +++ | + | + | + | + | ++ | - | + | + |
| DCM:MeOH | - | - | - | - | - | - | - | + | - | - | - | + | - | + | + | + | + | + | |
| 2LE-MW | - | - | - | - | + | + | - | ||||||||||||
| R-2LE-MW | - | - | + | - | + | + | + | ||||||||||||
| SFC1 | KOH ext | - | + | - | + | + | + | - | + | + | +++ | + | + | - | + | + | + | + | - |
| DCM:MeOH | - | + | - | - | - | - | - | + | + | - | - | - | - | + | +++ | + | - | - | |
| 2LE-MW | - | - | + | - | + | + | + | ||||||||||||
| R-2LE-MW | - | + | + | - | + | + | + | ||||||||||||
| SFC2 | KOH ext | - | + | - | - | - | - | - | + | + | + | - | - | - | + | - | + | - | - |
| DCM:MeOH | - | + | - | - | - | - | - | + | + | - | - | - | - | + | ++ | + | - | - | |
| 2LE-MW | - | - | - | - | + | + | - | ||||||||||||
| R-2LE-MW | - | - | + | - | + | + | - | ||||||||||||
| SFC3 | KOH ext | - | + | - | + | + | + | + | + | + | +++ | + | + | - | + | +++ | + | + | - |
| DCM:MeOH | - | + | - | + | - | - | - | + | + | - | - | - | - | + | +++ | + | - | + | |
| 2LE-MW | - | - | + | - | - | + | + | ||||||||||||
| R-2LE-MW | - | - | + | - | + | + | + | ||||||||||||
| SFC4 | KOH ext | - | + | - | - | - | - | - | + | + | + | - | - | - | + | ++ | + | + | + |
| DCM:MeOH | - | - | - | - | - | - | - | + | + | - | - | - | - | + | ++ | + | - | - | |
| 2LE-MW | - | - | - | - | + | + | - | ||||||||||||
| R-2LE-MW | - | - | + | - | + | + | - | ||||||||||||
| SFC5 | KOH ext | - | + | - | + | - | - | - | + | + | +++ | + | + | + | + | +++ | + | + | + |
| DCM:MeOH | - | + | - | - | - | - | - | + | + | - | - | - | - | + | ++ | + | - | + | |
| 2LE-MW | - | - | + | - | - | + | - | ||||||||||||
| R-2LE-MW | - | + | + | - | + | + | - | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chassouant, L.; Olmer, F.; Delpino, C.; Celant, A.; Vieillescazes, C.; Magri, D.; Mathe, C. Protocol Comparison for Organic Residue Analyses from Waterproofing Materials and Shards of Roman Archaeological Amphorae. Crystals 2021, 11, 1300. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111300
Chassouant L, Olmer F, Delpino C, Celant A, Vieillescazes C, Magri D, Mathe C. Protocol Comparison for Organic Residue Analyses from Waterproofing Materials and Shards of Roman Archaeological Amphorae. Crystals. 2021; 11(11):1300. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111300
Chicago/Turabian StyleChassouant, Louise, Fabienne Olmer, Chiara Delpino, Alessandra Celant, Cathy Vieillescazes, Donatella Magri, and Carole Mathe. 2021. "Protocol Comparison for Organic Residue Analyses from Waterproofing Materials and Shards of Roman Archaeological Amphorae" Crystals 11, no. 11: 1300. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111300