One-Step Formation of Reduced Graphene Oxide from Insulating Polymers Induced by Laser Writing Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. rGo from PLA Procedure for Conversion of the Polymers into rGO
2.2. Characterization
3. Results and Discussion
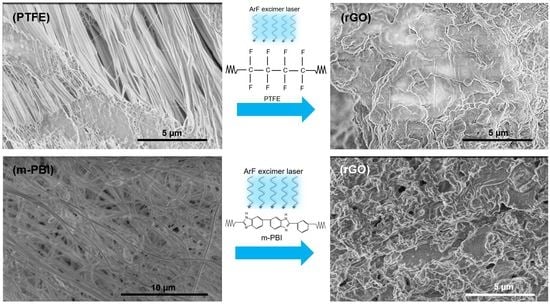
3.1. PTFE Conversion into rGO
3.1.1. X-Ray Diffraction
3.1.2. Scanning Electron Microscopy and Electron Backscattered Diffraction
3.1.3. Raman Spectroscopy
3.1.4. Fourier-Transform Infrared Spectroscopy
3.1.5. Mechanism of Conversion of PTFE into rGO by the PLA Method
3.2. PBI Conversion into rGO
3.2.1. X-ray Diffraction
3.2.2. Scanning Electron Microscopy
3.2.3. Raman Spectroscopy
3.2.4. Fourier-Transform Infrared Spectroscopy
3.2.5. Mechanism of Conversion of PBI into rGO by PLA Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2016, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 2009, 19, 3680–3695. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic Confinement and. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Song, Z.; Li, T.; Li, X.; Ogbazghi, A.Y.; Feng, R.; Dai, Z.; Alexei, N.; Conrad, M.E.H.; First, P.N.; et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 2004, 108, 19912–19916. [Google Scholar] [CrossRef] [Green Version]
- Rümmeli, M.H.; Bachmatiuk, A.; Scott, A.; Börrnert, F.; Warner, J.H.; Hoffman, V.; Lin, J.H.; Cuniberti, G.; Büchner, B. Direct low-temperature nanographene cvd synthesis over a dielectric insulator. ACS Nano 2010, 4, 4206–4210. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y., II; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; You, H.; Liu, F.; Li, M.; Wan, L.; Li, S.; Li, Q.; Xu, Y.; Tian, R.; Yu, Z.; et al. Large-scale synthesis of few-layered graphene using CVD. Chem. Vap. Depos. 2009, 15, 53–56. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Whitener, K.E.; Sheehan, P.E. Graphene synthesis. Diam. Relat. Mater. 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Lin, L.S.; Bin-Tay, W.; Li, Y.R.; Aslam, Z.; Westwood, A.; Brydson, R. A practical characterisation protocol for liquid-phase synthesised heterogeneous graphene. Carbon N. Y. 2020, 167, 307–321. [Google Scholar] [CrossRef]
- Yang, X.; Yan, M. Removing contaminants from transferred CVD graphene. Nano Res. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Dan, Y.; Lu, Y.; Kybert, N.J.; Luo, Z.; Johnson, A.T.C. Intrinsic response of graphene vapor sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, G.R.; Akhtar, F.; Ivanov, I.G.; Schmidt, S.; Shtepliuk, I.; Zakharov, A.; Iakimov, T.; Yakimova, R. Effect of epitaxial graphene morphology on adsorption of ambient species. Appl. Surf. Sci. 2019, 486, 239–248. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Direct laser writing of graphene electronics. ACS Nano 2014, 8, 8725–8729. [Google Scholar] [CrossRef] [PubMed]
- Spilarewicz-Stanek, K.; Kisielewska, A.; Ginter, J.; Bałuszyńska, K.; Piwoński, I. Elucidation of the function of oxygen moieties on graphene oxide and reduced graphene oxide in the nucleation and growth of silver nanoparticles. RSC Adv. 2016, 6, 60056–60067. [Google Scholar] [CrossRef] [Green Version]
- Orlita, M.; Faugeras, C.; Plochocka, P.; Neugebauer, P.; Martinez, G.; Maude, D.K.; Barra, A.L.; Sprinkle, M.; Berger, C.; De Heer, W.A.; et al. Approaching the dirac point in high-mobility multilayer epitaxial graphene. Phys. Rev. Lett. 2008, 101, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, P.R.; Narayan, R.J. Recent advances in carbon nanomaterials for biomedical applications: A review. Curr. Opin. Biomed. Eng. 2021, 17, 100262. [Google Scholar] [CrossRef]
- Sheng, K.; Sun, Y.; Li, C.; Yuan, W.; Shi, G. Ultrahigh-rate supercapacitors based on eletrochemically reduced graphene oxide for ac line-filtering. Sci. Rep. 2012, 2, 3–7. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, G.; Zhang, H.; Li, S. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coatings 2020, 147, 105741. [Google Scholar] [CrossRef]
- Abid; Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Hong, W.G.; Lee, S.M.; Chang, S.J.; Jun, Y.; Kim, B.H.; Kim, H.J. Energy storage of thermally reduced graphene oxide. Int. J. Hydrogen Energy 2014, 39, 3799–3804. [Google Scholar] [CrossRef]
- Iskandar, F.; Hikmah, U.; Stavila, E.; Aimon, A.H. Microwave-assisted reduction method under nitrogen atmosphere for synthesis and electrical conductivity improvement of reduced graphene oxide (rGO). RSC Adv. 2017, 7, 52391–52397. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamt, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Yan, L. Electrochemical reduction of bulk graphene oxide materials. RSC Adv. 2016, 6, 80106–80113. [Google Scholar] [CrossRef]
- Pousa Soares, C.P.; de Lacerda Baptista, R.; Cesar, D.V. Solvothermal reduction of graphite oxide using alcohols. Mater. Res. 2018, 21, 20170726. [Google Scholar] [CrossRef] [Green Version]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser Fabrication of Graphene-Based Flexible Electronics. Adv. Mater. 2020, 32, 1–22. [Google Scholar] [CrossRef]
- Narayan, J.; White, C.W. Pulsed laser melting of amorphous silicon layers. Appl. Phys. Lett. 1984, 44, 35–37. [Google Scholar] [CrossRef]
- Gupta, S. Structural Evolution of Novel Nonequilibrium Phases in Carbon and Boron Nitride via Liquid Phase Ultrafast Regrowth; North Carolina State University: Raleigh, NC, USA, 2020. [Google Scholar]
- Narayan, J.; Bhaumik, A.; Gupta, S.; Joshi, P.; Riley, P.; Narayan, R.J. Formation of self-organized nano- and micro-diamond rings. Mater. Res. Lett. 2021, 9, 300–307. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A.; Gupta, S.; Joshi, P.; Riley, P.; Narayan, R.J. Role of Q-carbon in nucleation and formation of continuous diamond film. Carbon N. Y. 2021, 176, 558–568. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5–12. [Google Scholar] [CrossRef]
- Ye, R.; Han, X.; Kosynkin, D.V.; Li, Y.; Zhang, C.; Jiang, B.; Martí, A.A.; Tour, J.M. Laser-Induced Conversion of Teflon into Fluorinated Nanodiamonds or Fluorinated Graphene. ACS Nano 2018, 12, 1083–1088. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light laser-induced graphene from phenolic resin: A new approach for directly writing graphene-based electrochemical devices on various substrates. Carbon N. Y. 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Lamberti, A.; Serrapede, M.; Ferraro, G.; Fontana, M.; Perrucci, F.; Bianco, S.; Chiolerio, A.; Bocchini, S. All-SPEEK flexible supercapacitor exploiting Laser-induced graphenization. 2D Mater. 2017, 4, 035012. [Google Scholar] [CrossRef]
- Joshi, P.; Riley, P.; Gupta, S.; Narayan, R.J.; Narayan, J. Advances in laser-assisted conversion of polymeric and graphitic carbon into nanodiamond films. Nanotechnology 2021, 32, 432001. [Google Scholar] [CrossRef]
- Schumann, M.; Sauerbrey, R.; Smayling, M.C. Permanent increase of the electrical conductivity of polymers induced by ultraviolet laser radiation. Appl. Phys. Lett. 1991, 58, 428–430. [Google Scholar] [CrossRef]
- Ptfe, T.; Teflon PTFE®. rjchase.com. Deels Teflon—PTFE | Deels. Available online: http://www.rjchase.com/ptfe_handbook.pdf (accessed on 23 September 2021).
- Penchev, H.; Ublekov, F.; Budurova, D.; Sinigersky, V. Novel electrospun polybenzimidazole fibers and yarns from ethanol/potassium hydroxide solution. Mater. Lett. 2017, 187, 89–93. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Grubova, I.Y.; Chernozem, R.V.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF magnetron sputtering of a hydroxyapatite target: A comparison study on polytetrafluorethylene and titanium substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Joshi, P.; Gupta, S.; Riley, P.R.; Narayan, R.J.; Narayan, J. Liquid phase regrowth of 〈110〉 nanodiamond film by UV laser annealing of PTFE to generate dense CVD microdiamond film. Diam. Relat. Mater. 2021, 117, 108481. [Google Scholar] [CrossRef]
- Lebedev, Y.A.; Korolev, Y.M.; Polikarpov, V.M.; Ignat’ev, L.N.; Antipov, E.M. X-Ray powder diffraction study of polytetrafluoroethylene. Crystallogr. Rep. 2010, 55, 609–614. [Google Scholar] [CrossRef]
- Hermans, P.H.; Weidinger, A. On the determination of the crystalline fraction of polyethylenes from X-ray diffraction. Die Makromol. Chem. 1961, 44, 24–36. [Google Scholar] [CrossRef]
- Sperati, C.A.; Starkweather, H.W. Fluorine-containing polymers. II. Polytetrafluoroethylene. In Fortschritte Der Hochpolymeren-Forschung 1961; Springer: Berlin/Heidelberg, Germany, 1961; pp. 465–495. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Lumban Raja, S.; Supeno, M.; Simanjuntak, C. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectros. Relat. Phenomena 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Mothudi, B.M.; Zorkani, I.; Jorio, A. X-ray diffraction investigations of nanostructured ZnO coated with reduced graphene oxide. J. Phys. Conf. Ser. 2019, 1292, 012011. [Google Scholar] [CrossRef]
- Ouederni, M. Polymers in textiles. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–363. [Google Scholar]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Koenig, J.L.; Boerio, F.J. Raman scattering and band assignments in polytetrafluoroethylene. J. Chem. Phys. 1969, 50, 2823–2829. [Google Scholar] [CrossRef]
- Schmälzlin, E.; Moralejo, B.; Rutowska, M.; Monreal-Ibero, A.; Sandin, C.; Tarcea, N.; Popp, J.; Roth, M.M. Raman imaging with a fiber-coupled multichannel spectrograph. Sensors (Switz.) 2014, 14, 21968–21980. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.T.; Pei, Y.T.; De Hosson, J.T.M. Structural changes in polytetrafluoroethylene molecular chains upon sliding against steel. J. Mater. Sci. 2014, 49, 1484–1493. [Google Scholar] [CrossRef]
- Lehnert, R.J.; Hendra, P.J.; Everall, N. Crystallinity of poly(tetrafluoroethylene) using Raman spectroscopy. Polymer 1995, 36, 2473–2476. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Rodriguez, R.D.; Ruban, A.; Pavlov, S.; Sheremet, E. The correlation between electrical conductivity and second-order Raman modes of laser-reduced graphene oxide. Phys. Chem. Chem. Phys. 2019, 21, 10125–10134. [Google Scholar] [CrossRef]
- Tuinstra, F. Koenig Jl Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Characterization techniques for graphene-based materials in catalysis. AIMS Mater. Sci. 2017, 4, 755–788. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Souza Filho, A.G.; Lieber, C.M.; Dresselhaus, m·s.; Pimenta, M.A.; Jorio, A.; Dresselhaus, G.; Hafner, J.H.; Saito, R. Diameter dependence of the Raman (formula presented)-band in isolated single-wall carbon nanotubes. Phys. Rev. B—Condens. Matter Mater. Phys. 2001, 64, 041401. [Google Scholar] [CrossRef]
- Haque, A.; Gupta, S.; Narayan, J. Characteristics of diamond deposition on Al2O3, diamond-like carbon, and Q-carbon. ACS Appl. Electron. Mater. 2020, 2, 1323–1334. [Google Scholar] [CrossRef]
- Shen, J.; Li, T.; Long, Y.; Shi, M.; Li, N.; Ye, M. One-step solid state preparation of reduced graphene oxide. Carbon N. Y. 2012, 50, 2134–2140. [Google Scholar] [CrossRef]
- Vollebregt, S.; Ishihara, R.; Tichelaar, F.D.; Hou, Y.; Beenakker, C.I.M. Influence of the growth temperature on the first and second-order Raman band ratios and widths of carbon nanotubes and fibers. Carbon N. Y. 2012, 50, 3542–3554. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Priya Rose, T.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400-700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Characterization of Graphite Fiber Surfaces with Raman Spectroscopy. J. Compos. Mater. 1970, 4, 492–499. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wen, Y.; Peng, H.; Zheng, C.; Li, Y.; Wang, S.; Sun, S.; Xie, X.; Zhou, X. Grafting polytetrafluoroethylene micropowder via in situ electron beam irradiation-induced polymerization. Polymers 2018, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, K.; Yao, Y.; Ochiai, T.; Sakai, M.; Kubota, Y.; Yamauchi, G. Antibacterial activity of hydrophobic composite materials containing a visible-light-sensitive photocatalyst. J. Nanotechnol. 2011, 2011, 380979. [Google Scholar] [CrossRef] [Green Version]
- Piwowarczyk, J.; Jedrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR studies of polytetrafluoroethylene thin films obtained by physical methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talonen, P.; Sundholm, G.; Li, W.H.; Floate, S.; Nichols, R.J. A combined in situ infrared spectroscopy and scanning tunnelling microscopy study of ethyl xanthate adsorption on Au(111). Phys. Chem. Chem. Phys. 1999, 1, 3661–3666. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Li, S.; Jiang, S.P. Self-assembled platinum nanoparticles on sulfonic acid-grafted graphene as effective electrocatalysts for methanol oxidation in direct methanol fuel cells. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.N.; Hur, S.H. One-step synthesis of a highly conductive graphene-polypyrrole nanofiber composite using a redox reaction and its use in gas sensors. Phys. Status Solidi—Rapid Res. Lett. 2012, 6, 379–381. [Google Scholar] [CrossRef]
- Bora, C.; Bharali, P.; Baglari, S.; Dolui, S.K.; Konwar, B.K. Strong and conductive reduced graphene oxide/polyester resin composite films with improved mechanical strength, thermal stability and its antibacterial activity. Compos. Sci. Technol. 2013, 87, 1–7. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376. [Google Scholar] [CrossRef]
- Dake, S.B.; Rajopadhye, N.R.; Bhoraskar, S. V Evaluation and characterisation of chemically modified polymers as secondary electron emitters. J. Phys. D Appl. Phys. 1987, 20, 1631–1636. [Google Scholar] [CrossRef]
- Herman, P.R.; Chen, B.; Moore, D.J. Vacuum ultraviolet laser ablation of Teflon (PTFE). In Proceedings of the Materials Research Society Symposium Proceedings; Cambridge University Press: Cambridge, UK, 1993; Volume 285, pp. 163–168. [Google Scholar]
- Bidin, N.; Ab Razak, S.N. ArF Excimer Laser Annealing of Polycrystalline Silicon Thin Film. In Crystallization—Science and Technology; InTech: London, UK, 2012. [Google Scholar]
- Ono, M.; Yamane, H.; Fukagawa, H.; Kera, S.; Yoshimura, D.; Okudaira, K.K.; Morikawa, E.; Seki, K.; Ueno, N. UPS study of VUV-photodegradation of polytetrafluoroethylene (PTFE) ultrathin film by using synchrotron radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 377–382. [Google Scholar] [CrossRef]
- Adami, M.; Guzman, L.; Man, B.Y.; Miotello, A.; Ossi, P.M. High temperature ion beam erosion of polytetrafluoroethylene. Thin Solid Films 2004, 459, 318–322. [Google Scholar] [CrossRef]
- Madorsky, S.I.; Hart, V.; Straus, S.; Sedlak, V.A. Thermal degradation of tetrafluoroethylene and hydrofluoroethylene polymers in a vacuum. J. Res. Natl. Bur. Stand. 1953, 51, 327. [Google Scholar] [CrossRef]
- Longo, A.; Verucchi, R.; Aversa, L.; Tatti, R.; Ambrosio, A.; Orabona, E.; Coscia, U.; Carotenuto, G.; Maddalena, P. Graphene oxide prepared by graphene nanoplatelets and reduced by laser treatment. Nanotechnology 2017, 28, 224002. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Z.; Jin, L.; Yang, Y.; Wang, S.; Yin, X.; Wang, Y. Pre-Oxidized Acrylic Fiber Reinforced Ferric Sulfophenyl Phosphate-Doped Polybenzimidazole-Based high-temperature proton exchange membrane. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Asadi Tashvigh, A.; Chung, T.S. Robust polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration. J. Memb. Sci. 2019, 572, 580–587. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, M.; Chen, N.; Niu, H.; Qi, S.; Wu, D. Fabrication of ultrahigh-strength polybenzimidazole fibers via a novel and green integrated liquid crystal spinning process. Macromol. Mater. Eng. 2020, 305, 1900717. [Google Scholar] [CrossRef]
- Daletou, M.K.; Geormezi, M.; Vogli, E.; Voyiatzis, G.A.; Neophytides, S.G. The interaction of H3PO4 and steam with PBI and TPS polymeric membranes. A TGA and Raman study. J. Mater. Chem. A 2014, 2, 1117–1127. [Google Scholar] [CrossRef]
- Conti, F.; Majerus, A.; Di Noto, V.; Korte, C.; Lehnert, W.; Stolten, D. Raman study of the polybenzimidazole-phosphoric acid interactions in membranes for fuel cells. Phys. Chem. Chem. Phys. 2012, 14, 10022–10026. [Google Scholar] [CrossRef] [Green Version]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B—Condens. Matter Mater. Phys. 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.R.; Joshi, P.; Azizi Machekposhti, S.; Sachan, R.; Narayan, J.; Narayan, R.J. Enhanced Vapor Transmission Barrier Properties via Silicon-Incorporated Diamond-Like Carbon Coating. Polymers 2021, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Haque, A.; Gupta, S.; Narayan, R.J.; Narayan, J. Synthesis of multifunctional microdiamonds on stainless steel substrates by chemical vapor deposition. Carbon N. Y. 2021, 171, 739–749. [Google Scholar] [CrossRef]
- Anandhan, S.; Ponprapakaran, K.; Senthil, T.; George, G. Parametric study of manufacturing ultrafine polybenzimidazole fibers by electrospinning. Int. J. Plast. Technol. 2012, 16, 101–116. [Google Scholar] [CrossRef]
- Jean-Fulcrand, A.; Masen, M.A.; Bremner, T.; Wong, J.S.S. Effect of temperature on tribological performance of polyetheretherketone-polybenzimidazole blend. Tribol. Int. 2019, 129, 5–15. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kumar, C.R.; Suresh, A.; Jayalakshmi, S.; Mudali, U.K.; Sivaraman, N. Evaluation of polybenzimidazole-based polymers for the removal of uranium, thorium and palladium from aqueous medium. R. Soc. Open Sci. 2018, 5, 171701. [Google Scholar] [CrossRef] [Green Version]
- Fei, M.; Lin, R.; Deng, Y.; Xian, H.; Bian, R.; Zhang, X.; Cheng, J.; Xu, C.; Cai, D. Polybenzimidazole/Mxene composite membranes for intermediate temperature polymer electrolyte membrane fuel cells. Nanotechnology 2018, 29, 035403. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, O.S.; Avadhani, C.V.; Singh, R.P. Photo-oxidative degradation of polybenzimidazole derivative membrane. Adv. Mater. Lett. 2013, 4, 762–768. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.R.; Joshi, P.; Narayan, J.; Narayan, R.J. Enhanced nucleation and large-scale growth of CVD diamond via surface-modification of silicon-incorporated diamond-like carbon thin films. Diam. Relat. Mater. 2021, 120, 108630. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Muthusamy, A.; Dineshkumar, S.; Kannapiran, N. Synthesis, characterization, optical, thermal and electrical properties of polybenzimidazoles. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 243–252. [Google Scholar] [CrossRef]
- Brown, J.R.; Burchill, P.J.; George, G.A.; Power, A.J. The photodegradation of poly 2,2′-(m-phenylene)-5,5′-bibenzimidazole. J. Polym. Sci. Polym. Symp. 2007, 49, 239–247. [Google Scholar] [CrossRef]
- Chatfield, D.A.; Einhorn, I.N. Stepwise thermal degradation of a polybenzimidazole foam. J. Polym. Sci. A 1981, 19, 601–618. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riley, P.R.; Joshi, P.; Penchev, H.; Narayan, J.; Narayan, R.J. One-Step Formation of Reduced Graphene Oxide from Insulating Polymers Induced by Laser Writing Method. Crystals 2021, 11, 1308. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111308
Riley PR, Joshi P, Penchev H, Narayan J, Narayan RJ. One-Step Formation of Reduced Graphene Oxide from Insulating Polymers Induced by Laser Writing Method. Crystals. 2021; 11(11):1308. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111308
Chicago/Turabian StyleRiley, Parand R., Pratik Joshi, Hristo Penchev, Jagdish Narayan, and Roger J. Narayan. 2021. "One-Step Formation of Reduced Graphene Oxide from Insulating Polymers Induced by Laser Writing Method" Crystals 11, no. 11: 1308. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111308