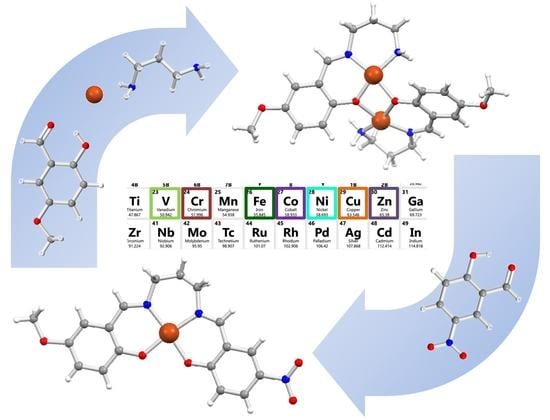
When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes
Abstract
:1. Introduction
2. Template Effect of Copper(II)
2.1. Tridentate N2O Schiff Bases and Their Omplexes
2.2. Unsymmetrically Substituted Salen-Type Copper(II) Complexes
3. Nickel (II) and Unsymmetrically Substituted Saltn Ligands
4. Template Effect of Other Metals
4.1. Iron(III), Chromium(III), and Cobalt(III)
(diamine = en, pn, chxn, dpen, tn)
4.2. Vanadium(V), Molibdenum(IV), and Tungsten(IV)
4.3. An Example of Template Method with Zinc(II) and Salophen Derivatives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindoy, L.F.; Busch, D.H. Metal Ion Controlled Syntheses of Novel Five-Coordinate Zinc and Cadmium Complexes Containing a Helical Coordination Geometry and Their Template Reaction to Form Complexes of a Pentadentate Macrocyclic Ligand. Inorg. Chem. 1974, 13, 2494–2498. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers. Nobel Lecture. Angew. Chem. Int. Ed. Engl. 1987, 27, 1021–1027. [Google Scholar] [CrossRef]
- Field, K.W.; Glover, A.D.; Moroz, J.S.; Collander, D.J.; Kolb, K.E. “Crown Ether” Synthesis: An Organic Laboratory Experiment. J. Chem. Educ. 1979, 56, 269. [Google Scholar] [CrossRef]
- Glueck, D.S.; Brough, A.R.; Mountford, P.; Green, M.L.H. Synthesis and Properties of Crown Ether-Alkali Metal Cation Intercalation Compounds of Transition Metal Phosphorus Sulfides, MPS3 (M = Manganese, Cadmium, Zinc). Inorg. Chem. 1993, 32, 1893–1902. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, 5th ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-44897-8. [Google Scholar]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Ann. Chem. Pharm. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Vigato, P.; Tamburini, S.; Bertolo, L. The Development of Compartmental Macrocyclic Schiff Bases and Related Polyamine Derivatives. Coord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- Frischmann, P.D.; MacLachlan, M.J. Schiff Base Macrocycles: Reliable Templates for Multinuclear Metallocavitands. Comments Inorg. Chem. 2008, 29, 26–45. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. Acyclic and Cyclic Compartmental Ligands: Recent Results and Perspectives. Coord. Chem. Rev. 2012, 256, 953–1114. [Google Scholar] [CrossRef]
- Rezaeivala, M.; Keypour, H. Schiff Base and Non-Schiff Base Macrocyclic Ligands and Complexes Incorporating the Pyridine Moiety—The First 50 Years. Coord. Chem. Rev. 2014, 280, 203–253. [Google Scholar] [CrossRef]
- Gavey, E.L.; Pilkington, M. Coordination Complexes of 15-Membered Pentadentate Aza, Oxoaza and Thiaaza Schiff Base Macrocycles “Old Complexes Offer New Attractions”. Coord. Chem. Rev. 2015, 296, 125–152. [Google Scholar] [CrossRef]
- Akine, S. Metal Complexes with Oligo(Salen)-Type Ligands. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–42. ISBN 978-0-470-68253-1. [Google Scholar]
- Radecka-Paryzek, W.; Patroniak, V.; Lisowski, J. Metal Complexes of Polyaza and Polyoxaaza Schiff Base Macrocycles. Coord. Chem. Rev. 2005, 249, 2156–2175. [Google Scholar] [CrossRef]
- Riley, D.P.; Busch, D.H.; Fenton, D.E.; Lintvedt, R.L. Macrocyclic Tetraazatetraenato Ligands and their Metal Complexes. In Inorganic Syntheses; Douglas, B.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 36–44. ISBN 978-0-470-13249-4. [Google Scholar]
- Golbedaghi, R.; Tabanez, A.M.; Esmaeili, S.; Fausto, R. Biological Applications of Macrocyclic Schiff Base Ligands and Their Metal Complexes: A Survey of the Literature (2005–2019). Appl. Organomet. Chem. 2020, 34, e5884. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The Challenge of Cyclic and Acyclic Schiff Bases and Related Derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.-R. Recent Developments in Penta-, Hexa- and Heptadentate Schiff Base Ligands and Their Metal Complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Akine, S.; Nabeshima, T. Cyclic and Acyclic Oligo(N2O2) Ligands for Cooperative Multi-Metal Complexation. Dalton Trans. 2009, 10395. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jones, R.A.; Huang, S. Luminescent 4f and d-4f Polynuclear Complexes and Coordination Polymers with Flexible Salen-Type Ligands. Coord. Chem. Rev. 2014, 273–274, 63–75. [Google Scholar] [CrossRef]
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. REVIEW: Biomedical Applications of Schiff Base Metal Complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Cotton, F.A. Advanced Inorganic Chemistry; Wiley: New Delhi, India, 2008; ISBN 978-81-265-1338-3. [Google Scholar]
- Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. Tricyclische orthokondensierte Nebenvalenzringe. Justus Liebig’s Ann. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Pasini, A.; Demartin, F.; Piovesana, O.; Chiari, B.; Cinti, A.; Crispu, O. Novel Copper(II) Complexes of “Short” Salen Homologues. Structure and Magnetic Properties of the Tetranuclear Complex [Cu2(L2)2]2 [H2L2 = Phenyl-N,N′-Bis(Salicylidene)Methanediamine]. J. Chem. Soc. Dalton Trans. 2000, 3467–3472. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. Binuclear Co(II)Co(II), Co(II)Co(III) and Co(III)Co(III) Complexes of “Short” Salen Homologues Derived from the Condensation of Salicylaldehyde and Methanediamine or Phenylmethanediamines. Synthesis, Structures and Magnetism. J. Chem. Soc. Dalton Trans. 2001, 3611–3616. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. New Pentanuclear Mixed Valence Co(ii)–Co(iii) Complexes of “Short” Salen Homologues. J. Chem. Soc., Dalton Trans. 2002, 4672–4677. [Google Scholar] [CrossRef]
- Novitchi, G.; Shova, S.; Cascaval, A.; Gulea, A. Synthesis, Structure and Complex Formation of N,N’-Disalicylidenemethylendiamine (Salmen). Revue Roumanie de Chimie 2002, 47, 1027–1035. [Google Scholar]
- Rigamonti, L.; Zardi, P.; Carlino, S.; Demartin, F.; Castellano, C.; Pigani, L.; Ponti, A.; Ferretti, A.M.; Pasini, A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 7882. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Yamamura, M. Cooperative Formation and Functions of Multimetal Supramolecular Systems. Pure Appl. Chem. 2013, 85, 763–776. [Google Scholar] [CrossRef]
- Hobday, M.D.; Smith, T.D. N,N’-Ethylenebis(Salicylideneiminato) Transition Metal Ion Chelates. Coord. Chem. Rev. 1973, 9, 311–337. [Google Scholar] [CrossRef]
- Yoon, T.P. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Erxleben, A. Transition Metal Salen Complexes in Bioinorganic and Medicinal Chemistry. Inorg. Chim. Acta 2018, 472, 40–57. [Google Scholar] [CrossRef]
- Weber, B.; Jäger, E.-G. Structure and Magnetic Properties of Iron(II/III) Complexes with N2O22- Coordinating Schiff Base Like Ligands. Eur. J. Inorg. Chem. 2009, 2009, 465–477. [Google Scholar] [CrossRef]
- Kleij, A.W. New Templating Strategies with Salen Scaffolds (Salen = N,N′-Bis(Salicylidene)Ethylenediamine Dianion). Chem. Eur. J. 2008, 14, 10520–10529. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.M.; Herasymchuk, K.; Storr, T. Electronic Structure Elucidation in Oxidized Metal–Salen Complexes. Coord. Chem. Rev. 2017, 352, 67–82. [Google Scholar] [CrossRef]
- Wei, F.Y.; Wen, P.H. Synthesis, Structures, and Antibacterial Activities of Two Iron(III) Complexes with Schiff Bases. Russ. J. Coord. Chem. 2014, 40, 289–296. [Google Scholar] [CrossRef]
- Elder, R.C. Tridentate and Unsymmetrical Tetradentate Schiff Base Ligands from Salicylaldehydes and Diamines: Their Monomeric and Dimeric Nickel(II) Complexes. Aus. J. Chem. 1978, 31, 35–45. [Google Scholar] [CrossRef]
- Gomes, L.; Pereira, E.; de Castro, B. Nickel(II) Complexes with N2OS and N2S2 Coordination Spheres: Reduction and Spectroscopic Study of the Corresponding Ni(I) Complexes. J. Chem. Soc. Dalton Trans. 2000, 1373–1379. [Google Scholar] [CrossRef]
- Rigamonti, L.; Cinti, A.; Forni, A.; Pasini, A.; Piovesana, O. Copper(II) Complexes of Tridentate Schiff Bases of 5-Substituted Salicylaldehydes and Diamines—The Role of the Substituent and the Diamine in the Formation of Mono-, Di- and Trinuclear Species—Crystal Structures and Magnetic Properties. Eur. J. Inorg. Chem. 2008, 2008, 3633–3647. [Google Scholar] [CrossRef]
- Kleij, A.W. Nonsymmetrical Salen Ligands and Their Complexes: Synthesis and Applications. Eur. J. Inorg. Chem. 2009, 2009, 193–205. [Google Scholar] [CrossRef]
- Lopez, J.; Liang, S.; Bu, X.R. Unsymmetric Chiral Salen Schiff Bases: A New Chiral Ligand Pool from Bis-Schiff Bases Containing Two Different Salicylaldehyde Units. Tetrahedron Lett. 1998, 39, 4199–4202. [Google Scholar] [CrossRef]
- Lopez, J.; Mintz, E.A.; Hsu, F.-L.; Bu, X.R. Novel Unsymmetric Chiral Schiff Bases Possessing Two Different Donor Moieties: Unique Tetradentate Ligands from Combination of Salicylaldehyde and Acetylacetone Units. Tetrahedron Asymm. 1998, 9, 3741–3744. [Google Scholar] [CrossRef]
- Renehan, M.F.; Schanz, H.-J.; McGarrigle, E.M.; Dalton, C.T.; Daly, A.M.; Gilheany, D.G. Unsymmetrical Chiral Salen Schiff Base Ligands. J. Mol. Cat. A Chem. 2005, 231, 205–220. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Maleev, V.I.; North, M.; Larionov, V.A.; Savel’yeva, T.F.; Nijland, A.; Nelyubina, Y.V. Chiral Octahedral Complexes of CoIII As a Family of Asymmetric Catalysts Operating under Phase Transfer Conditions. ACS Catal. 2013, 3, 1951–1955. [Google Scholar] [CrossRef]
- Lalehzari, A.; Desper, J.; Levy, C.J. Double-Stranded Monohelical Complexes from an Unsymmetrical Chiral Schiff-Base Ligand. Inorg. Chem. 2008, 47, 1120–1126. [Google Scholar] [CrossRef]
- Muñoz-Hernández, M.-A.; Keizer, T.S.; Parkin, S.; Patrick, B.; Atwood, D.A. Group 13 Cation Formation with a Potentially Tridentate Ligand. Organometallics 2000, 19, 4416–4421. [Google Scholar] [CrossRef]
- Kleij, A.W.; Tooke, D.M.; Spek, A.L.; Reek, J.N.H. A Convenient Synthetic Route for the Preparation of Nonsymmetric Metallo-Salphen Complexes. Eur. J. Inorg. Chem. 2005, 2005, 4626–4634. [Google Scholar] [CrossRef] [Green Version]
- Boghaei, D.M.; Mohebi, S. Non-Symmetrical Tetradentate Vanadyl Schiff Base Complexes Derived from 1,2-Phenylene Diamine and 1,3-Naphthalene Diamine as Catalysts for the Oxidation of Cyclohexene. Tetrahedron 2002, 58, 5357–5366. [Google Scholar] [CrossRef]
- Johnson, M.S.; Horton, C.L.; Gonawala, S.; Verani, C.N.; Metzger, R.M. Observation of Current Rectification by a New Asymmetric Iron(iii) Surfactant in a Eutectic GaIn|LB Monolayer|Au Sandwich. Dalton Trans. 2018, 47, 6344–6350. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, M.J.; Park, M.K.; Thompson, L.K. Coordination Compounds of Schiff-Base Ligands Derived from Diaminomaleonitrile (DMN): Mononuclear, Dinuclear, and Macrocyclic Derivatives. Inorg. Chem. 1996, 35, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Varsha, G.; Arun, V.; Sebastian, M.; Leeju, P.; Varghese, D.; Yusuff, K.K.M. (Z)-2-Amino-3-[(E)-Benzylideneamino]- but-2-Enedinitrile. Acta Crystallogr. E 2009, 65, o919. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Lamère, J.F.; Lepetit, C.; Lacroix, P.G.; Dahan, F.; Nakatani, K. Synthesis, Crystal Structures, and Nonlinear Optical (NLO) Properties of New Schiff-Base Nickel(II) Complexes. Toward a New Type of Molecular Switch? Inorg. Chem. 2005, 44, 1973–1982. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Bridging Ability of a Novel Polydentate Ligand (H2L) Comprising an Oxime Function. Structures of a Mononuclear Precursor [NiL] and a Dinuclear CuII2 Complex. Magnetic Properties of Mononuclear (NiII and CuII), Dinuclear (CuII2, NiII2, NiIICuII and CuIICrIII) and Trinuclear (CuII3, CuIIMnIICuII and CuIIZnIICuII) Complexes. J. Chem. Soc. Dalton Trans. 1998, 1307–1314. [Google Scholar] [CrossRef]
- Costes, J.-P.; Cros, G.; Darbieu, M.-H.; Laurent, J.-P. The Non-Template Synthesis of Novel Non-Symmetrical, Tetradentate Schiff Bases. Their Nickel(II) and Cobalt(III) Complexes. Inorg. Chim. Acta 1982, 60, 111–114. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Kwiatkowski, E.; Olechnowicz, A.; Ho, D.M.; Deutsch, E. A Convenient Synthetic Route to the Monocondensation Products of Pentane-2,4-Dione and Aliphatic α,ω-Diamines. Synthesis, X-Ray Structure and Magnetic Properties of a Trinuclear Copper(II) Complex with 8-Amino-5-Aza-4-Methyl-3-Octene-2-One. Inorg. Chim. Acta 1988, 150, 65–73. [Google Scholar] [CrossRef]
- Trujillo, A.; Fuentealba, M.; Carrillo, D.; Manzur, C.; Hamon, J.-R. Synthesis, Characterization and X-Ray Crystal Structure of an Allyloxo Functionalized Nonsymmetric Nickel Coordination Complex Based on N2O2 Chelating Ferrocenyl Ligand. J. Organomet. Chem. 2009, 694, 1435–1440. [Google Scholar] [CrossRef]
- Trujillo, A.; Fuentealba, M.; Carrillo, D.; Manzur, C.; Ledoux-Rak, I.; Hamon, J.-R.; Saillard, J.-Y. Synthesis, Spectral, Structural, Second-Order Nonlinear Optical Properties and Theoretical Studies On New Organometallic Donor−Acceptor Substituted Nickel(II) and Copper(II) Unsymmetrical Schiff-Base Complexes. Inorg. Chem. 2010, 49, 2750–2764. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ray, M.S.; Chaudhuri, S.; Mukhopadhyay, G.; Bocelli, G.; Cantoni, A.; Ghosh, A. Nickel(II) and Copper(II) Complexes of Tetradentate Unsymmetrical Schiff Base Ligands: First Evidence of Positional Isomerism in Such System. Inorg. Chim. Acta 2006, 359, 1367–1375. [Google Scholar] [CrossRef]
- Bhowmik, P.; Drew, M.G.B.; Chattopadhyay, S. Synthesis and Characterization of Nickel(II) and Copper(II) Complexes with Tetradentate Schiff Base Ligands. Inorg. Chim. Acta 2011, 366, 62–67. [Google Scholar] [CrossRef]
- Matsumoto, N.; Yamashita, S.; Ohyoshi, A.; Kohata, S.; Ōkawa, H. Synthesis and X-Ray Crystal Structures of an Imidazolate-Bridged Polynuclear Copper(II) Complex Exhibiting a Unique Helicoid Structure and Its Precursor Mononuclear Complex. J. Chem. Soc. Dalton Trans. 1988, 1943–1948. [Google Scholar] [CrossRef]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Expedient Method for the Transmetalation of Zn(II)-Centered Salphen Complexes. Inorg. Chem. 2007, 46, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P. Complexes Du Cuivre(II) Avec l’(Hydroxy-2-Phényl)-4-Aza-Butene-3-Amine-1 et Molécules Dérivées. Bull. Chem. Soc. France 1986, 1, 78–82. [Google Scholar]
- Rigamonti, L.; Demartin, F.; Forni, A.; Righetto, S.; Pasini, A. Copper(II) Complexes of Salen Analogues with Two Differently Substituted (Push−Pull) Salicylaldehyde Moieties. A Study on the Modulation of Electronic Asymmetry and Nonlinear Optical Properties. Inorg. Chem. 2006, 45, 10976–10989. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Pievo, R.; Reedijk, J.; Pasini, A. Copper(II) Compounds with NNO Tridentate Schiff Base Ligands: Effect of Subtle Variations in Ligands on Complex Formation, Structures and Magnetic Properties. Inorg. Chim. Acta 2012, 387, 373–382. [Google Scholar] [CrossRef]
- Fernandez Garcia, M.I.; Fondo, M.; Garcia Deibe, A.M.; Fernandez Fernandez, M.B.; Gonzalez, A.M. Copper(II) Complexes with Asymmetrical Schiff Base Ligands Derived from 2-Acetylpyrazine. Z. Anorg. Allg. Chem. 2000, 626, 1985–1991. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Fernandez Fernandez, M.B.; Fernandez Garcia, M.I.; Garcia Deibe, A.M.; Sanmartin, J. General Synthesis of ‘Salicylaldehyde Half-Unit Complexes’: Structural Determination and Use as Synthon for the Synthesis of Dimetallic or Trimetallic Complexes and of ‘Self-Assembling Ligand Complexes’. Inorg. Chim. Acta 1998, 274, 73–81. [Google Scholar] [CrossRef]
- Costes, J.-P.; Chiboub Fellah, F.Z.; Dahan, F.; Duhayon, C. Role of the Kinetic Template Effect in the Syntheses of Non Symmetric Schiff Base Complexes. Polyhedron 2013, 52, 1065–1072. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Cariati, E.; Malavasi, G.; Pasini, A. Solid-State Nonlinear Optical Properties of Mononuclear Copper(II) Complexes with Chiral Tridentate and Tetradentate Schiff Base Ligands. Materials 2019, 12, 3595. [Google Scholar] [CrossRef] [Green Version]
- Fellah, F.Z.C.; Costes, J.-P.; Dahan, F.; Duhayon, C.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- and Triheteronuclear Cu−Gd and Cu−Gd−Cu Complexes with Dissymmetric Double Bridge. Inorg. Chem. 2008, 47, 6444–6451. [Google Scholar] [CrossRef] [PubMed]
- Behzad, M.; Seifikar Ghomi, L.; Damercheli, M.; Mehravi, B.; Shafiee Ardestani, M.; Samari Jahromi, H.; Abbasi, Z. Crystal Structures and in Vitro Anticancer Studies on New Unsymmetrical Copper(II) Schiff Base Complexes Derived from Meso-1,2-Diphenyl-1,2-Ethylenediamine: A Comparison with Related Symmetrical Ones. J. Coord. Chem. 2016, 69, 2469–2481. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.K.; Nag, K. Synthesis of Phenoxo-Bridged Dicopper(II) Complexes of N-(2-Aminoalkyl)Salicylaldimines and Their Use in the Formation of Monohalogeno-Complexes and Non-Symmetrical Quadridentate Schiff-Base Complexes. J. Chem. Soc. Dalton Trans. 1984, 2839–2841. [Google Scholar] [CrossRef]
- Leluk, M.; Jeżowska-Trzebiatowska, B.; Jezierska, J. Magnetic and ESR Studies of Copper(II) Complexes with N-(Aminoalkyl)Salicylaldimines. Polyhedron 1991, 10, 1653–1656. [Google Scholar] [CrossRef]
- Paschke, R.; Balkow, D.; Sinn, E. Lowering Melting Points in Asymmetrically Substituted Salen-Copper(II) Complexes Exhibiting Mesomorphic Behavior. Structure of the Mesogen Cu(5-HexyloxySalen). Inorg. Chem. 2002, 41, 1949–1953. [Google Scholar] [CrossRef]
- Ray, M.S.; Mukhopadhyay, G.; Drew, M.G.B.; Lu, T.-H.; Chaudhuri, S.; Ghosh, A. A Diphenoxo Bridged Antiferromagnetically Coupled Dimer of Copper(II) Having Bridging Methanol. Inorg. Chem. Commun. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Sironi, M.; Ponti, A.; Ferretti, A.M.; Baschieri, C.; Pasini, A. Experimental and Theoretical Investigations on Magneto-Structural Correlation in Trinuclear Copper(II) Hydroxido Propellers. Polyhedron 2018, 145, 22–34. [Google Scholar] [CrossRef]
- Bian, H.-D.; Xu, J.-Y.; Gu, W.; Yan, S.-P.; Cheng, P.; Liao, D.-Z.; Jiang, Z.-H. Synthesis, X-Ray Structure and Magnetic Properties of Trinuclear Copper(II) Tridentate Schiff Base Complexes Containing a Partial Cubane Cu3O4 Core. Polyhedron 2003, 22, 2927–2932. [Google Scholar] [CrossRef]
- Chiboub Fellah, F.Z.; Costes, J.-P.; Vendier, L.; Duhayon, C.; Ladeira, S.; Tuchagues, J.-P. μ3—vs. μ-Hydroxido Bridges—Peripheral Function Controls the Nuclearity of Hydroxido-Bridged Copper(II) Complexes. Eur. J. Inorg. Chem. 2012, 2012, 5729–5740. [Google Scholar] [CrossRef]
- Bhowmik, P.; Jana, S.; Mahapatra, P.; Giri, S.; Chattopadhyay, S.; Ghosh, A. Role of Steric Crowding of Ligands in the Formation of Hydroxido Bridged Di- and Trinuclear Copper(II) Complexes: Structures and Magnetic Properties. Polyhedron 2018, 145, 43–52. [Google Scholar] [CrossRef]
- Biswas, C.; Drew, M.G.B.; Figuerola, A.; Gómez-Coca, S.; Ruiz, E.; Tangoulis, V.; Ghosh, A. Magnetic Coupling in Trinuclear Partial Cubane Copper(II) Complexes with a Hydroxo Bridging Core and Peripheral Phenoxo Bridges from NNO Donor Schiff Base Ligands. Inorg. Chim. Acta 2010, 363, 846–854. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Pievo, R.; Reedijk, J.; Pasini, A. Synthesis, Crystal Structures and Magnetic Properties of Dinuclear Copper(II) Compounds with NNO Tridentate Schiff Base Ligands and Bridging Aliphatic Diamine and Aromatic Diimine Linkers. Dalton Trans. 2011, 40, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, O.; Chiari, B.; Cinti, A.; Sulpice, A. Synthesis, Structure, and Magnetic Properties of Cu2L2Cl2 (LH = N-Salicylidene-1,2-Ethanediamine)—A New S = 1/2 Spin-Liquid Candidate. Eur. J. Inorg. Chem. 2011, 2011, 4414–4420. [Google Scholar] [CrossRef]
- Bhowmik, P.; Jana, S.; Chattopadhyay, S. Anion Directed Templated Synthesis of Mono- and Di-Condensed Schiff Base Compounds of Cu(II). Polyhedron 2012, 44, 11–17. [Google Scholar] [CrossRef]
- Bhowmik, P.; Bhattacharyya, A.; Harms, K.; Sproules, S.; Chattopadhyay, S. Anion Directed Cation Templated Synthesis of Three Ternary Copper(II) Complexes with a Monocondensed N2O Donor Schiff Base and Different Pseudohalides. Polyhedron 2015, 85, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Verma, A.; Bretosh, K.; Sutter, J.-P.; Sunkari, S.S. Template Directed Synthesis of Half Condensed Schiff Base Complexes of Cu(II) and Co(III): Structural and Magnetic Studies. Polyhedron 2019, 164, 80–89. [Google Scholar] [CrossRef]
- Rigamonti, L.; Reginato, F.; Ferrari, E.; Pigani, L.; Gigli, L.; Demitri, N.; Kopel, P.; Tesarova, B.; Heger, Z. From Solid State to in Vitro Anticancer Activity of Copper(II) Compounds with Electronically-Modulated NNO Schiff Base Ligands. Dalton Trans. 2020, 49, 14626–14639. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Drew, M.G.B.; Lu, C.-Z.; Tercero, J.; Diaz, C.; Ghosh, A. Dinuclear Complexes of MII Thiocyanate (M = Ni and Cu) Containing a Tridentate Schiff-Base Ligand: Synthesis, Structural Diversity and Magnetic Properties. Eur. J. Inorg. Chem. 2005, 2005, 2376–2383. [Google Scholar] [CrossRef]
- Sain, S.; Maji, T.K.; Das, D.; Cheng, J.; Lu, T.-H.; Ribas, J.; El Fallah, M.S.; Chaudhuri, N.R. Exchange Interactions in a One-Dimensional Bromo-Bridged Copper(II) Compound with a Ladder-like Structure. J. Chem. Soc. Dalton Trans. 2002, 1302–1306. [Google Scholar] [CrossRef]
- Béreau, V.; Dhers, S.; Costes, J.-P.; Duhayon, C.; Sutter, J.-P. Syntheses, Structures, and Magnetic Properties of Symmetric and Dissymmetric Ester-Functionalized 3d-4f Schiff Base Complexes: Syntheses, Structures, and Magnetic Properties of Symmetric and Dissymmetric Ester-Functionalized 3d-4f Schiff Base Complexes. Eur. J. Inorg. Chem. 2018, 2018, 66–73. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A. Influence of Anionic Ligands (X) on the Nature and Magnetic Properties of Dinuclear LCuGdX3·nH2O Complexes (LH2 Standing for Tetradentate Schiff Base Ligands Deriving from 2-Hydroxy-3-Methoxybenzaldehyde and X Being Cl, N3C2, and CF3COO). Inorg. Chem. 2000, 39, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Freire, C.; De Castro, B. Synthesis, Spectroscopic and Electrochemical Characteriisation of Nickel Complexes with Two N2O Tridentate, Unsymmetrical Schiff Base Ligands. J. Coord. Chem. 2001, 54, 1–12. [Google Scholar] [CrossRef]
- Sousa, C.; Gameiro, P.; Freire, C.; de Castro, B. Nickel(II) and Copper(II) Schiff Base Complexes Bearing Benzo-15-Crown-5 Functionalities as Probes for Spectroscopic Recognition of Lanthanide Ions. Polyhedron 2004, 23, 1401–1408. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nandi, M.; Mayer-Figge, H.; Sheldrick, W.S.; Sorace, L.; Bhaumik, A.; Banerjee, P. Nickel Complexes with N2O Donor Ligands: Syntheses, Structures, Catalysis and Magnetic Studies. Eur. J. Inorg. Chem. 2007, 2007, 5033–5044. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Righetto, S.; Pasini, A. Push-Pull Unsymmetrical Substitution in Nickel(II) Complexes with Tetradentate N2O2 Schiff Base Ligands: Synthesis, Structures and Linear-Nonlinear Optical Studies. Dalton Trans. 2019, 48, 11217–11234. [Google Scholar] [CrossRef]
- Tahir, M.N.; Ülkü, D.; Nazir, H.; Atakol, O. Bis{2-[(3-Aminopropyl)Iminomethyl]-4,6-Dinitrophenolato- O,N,N’}nickel(II). Acta Crystallogr. C 1997, 53, 181–183. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Drew, M.G.B.; Ghosh, A. Anion Directed Templated Synthesis of Mono- and Di-Schiff Base Complexes of Ni(II). Polyhedron 2007, 26, 3513–3522. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Ghosh, A. Anion-Directed Template Synthesis and Hydrolysis of Mono-Condensed Schiff Base of 1,3-Pentanediamine and o-Hydroxyacetophenone in NiII and CuII Complexes. Eur. J. Inorg. Chem. 2008, 2008, 3372–3381. [Google Scholar] [CrossRef]
- Sacconi, L.; Nardi, N.; Zanobini, F. Complexes of Nickel(II) with Schiff Bases Formed from Salicyalaldehydes and N-Substituted Trimethylenediamines. Inorg. Chem. 1966, 5, 1872–1876. [Google Scholar] [CrossRef]
- Root, C.A.; Rising, B.A.; VanDerveer, M.C.; Hellmuth, C.F. New Ring-Opening Reaction of Aziridines in Nickel(II) and Copper(II) Complexes. Inorg. Chem. 1972, 11, 1489–1493. [Google Scholar] [CrossRef]
- Nozaki, T.; Ushio, H.; Mago, G.; Matsumoto, N.; Ōkawa, H.; Yamakawa, Y.; Anno, T.; Nakashima, T. Ligand-Field Control in the Self-Assembly of Polymeric Metal Complexes: Copper(II) Complexes with Quadridentate Schiff-Base Ligands Involving an Imidazole Moiety. J. Chem. Soc. Dalton Trans. 1994, 2339–2345. [Google Scholar] [CrossRef]
- Nozaki, T.; Matsumoto, N.; Okawa, H.; Miyasaka, H.; Mago, G. B-N Bond Formation by the Reaction of (N-(3-Methoxysalicylidene)-N’-(Imidazol-4-Ylmethylene)-1,3-Propanediamino)Copper(II) Perchlorate and Sodium Tetraphenylborate. Inorg. Chem. 1995, 34, 2108–2112. [Google Scholar] [CrossRef]
- Mimura, M.; Matsuo, T.; Matsumoto, N.; Takamizawa, S.; Mori, W.; Re, N. Bis[(Methanol){N-Salicylidene-N′-(2-Phenylimidazol-4-Ylmethylidene)-1,3-Propanediaminato}nickel(II)] Bridged by Di-μ-Phenoxo Moiety and the Deprotonated Imidazolate-Bridged Cyclic-Tetranuclear Complex. Bull. Chem. Soc. Jpn. 1998, 71, 1831–1837. [Google Scholar] [CrossRef]
- Kr. Sen, S. Unsymmetrical Tetradentate Ligands and Its Metal Chelates: I. Copper(II) and Nickel(II) Complexes with 1∶1∶1 Condensate Derived from β-Hydroxy-α-Napthaldehyde, 1,3-Propanediamine and Different Aldehydes. Trans. Met. Chem. 1983, 8, 75–78. [Google Scholar] [CrossRef]
- Burke, P.J.; McMillin, D.R. Unsymmetrical Ligand Complexes of CuII, NiII, and CoII Derived from Salicylaldehyde and 1,3-Propanediamine with Either Pyridine-2-Carbaldehyde or Pyrrole-2-Carbaldehyde. J. Chem. Soc. Dalton Trans. 1980, 1794. [Google Scholar] [CrossRef]
- Matsumoto, N.; Mimura, M.; Sunatsuki, Y.; Eguchi, S.; Mizuguchi, Y.; Miyasaka, H.; Nakashima, T. Steric-Hindrance Effect of a Substituent in the Self-Assembly Process of Copper(II) Complexes with Quadridentate Schiff-Base Ligands Involving a 2-Substituted-Imidazole Moiety. Bull. Chem. Soc. Japan 1997, 70, 2461–2472. [Google Scholar] [CrossRef]
- Mahapatra, P.; Ghosh, S.; Giri, S.; Ghosh, A. The Unusual Intermediate Species in the Formation of Ni(II) Complexes of Unsymmetrical Schiff Bases by Elder’s Method: Structural, Electrochemical and Magnetic Characterizations. Polyhedron 2016, 117, 427–436. [Google Scholar] [CrossRef]
- Fallon, G.D.; Gatehouse, B.M. Crystal and Molecular Structure of an Octahedral Iron(III) Complex with a Sulphur-Containing Schiff-Base Ligand: Bis(2-Aminoethylthiosalicylideneiminato)Iron(III) Chloride. J. Chem. Soc. Dalton Trans. 1975, 1344. [Google Scholar] [CrossRef]
- Summerton, A.P.; Diamantis, A.A.; Snow, M.R. The Crystal Structure of Bis[N-(2-Aminoethyl) Salicylaldiminato]Iron(III) Chloride Monohydrate, a Low Spin Iron(III) Complex Stabilized by Lattice Water. Inorg. Chim. Acta 1978, 27, 123–128. [Google Scholar] [CrossRef]
- Feng, X.; Han, X.; Wang, L.-Y. Crystal Structure of Bis(N-(2-Aminoethyl)Salicylaldiminato)Iron(III) Isothiocyanide, [Fe(C9H11N2O)2][NCS]. Z. Kristallogr. NCS 2006, 221. [Google Scholar] [CrossRef]
- Basak, T.; Ghosh, K.; Chattopadhyay, S. Synthesis, Characterization and Catechol Oxidase Mimicking Activity of Two Iron(III) Schiff Base Complexes. Polyhedron 2018, 146, 81–92. [Google Scholar] [CrossRef]
- Gardner, A.P.; Gatehouse, B.M.; White, J.V.B. Structure of Bis-(2-Aminoethylsalicylideneiminato) Chromium(III) Iodide. Chem. Commun. 1968, 694–695. [Google Scholar] [CrossRef]
- O’Connor, M.J.; West, B.O. N-Substituted Salicylaldimine Complexes of Chromium(III). Aus. J. Chem. 1968, 21, 369–372. [Google Scholar] [CrossRef]
- Gardner, A.P.; Gatehouse, B.M.; White, J.C.B. The Crystal Structure of Bis-(2-Aminoethylsalicylideneiminato)Chromium(III) Iodide. Acta Crystallogr. B 1971, 27, 1505–1509. [Google Scholar] [CrossRef]
- Bilton, M.S. (-)409-R,S-[(R-N(2-Aminopropyl)salicylaldiminato)chromium(III)]perchlorate, [C20H26N4O2Cr]+ClO4-. Cryst. Struct. Commun. 1982, 11, 755–762. [Google Scholar]
- Biswas, S.; Sarkar, S.; Dey, K.; Jana, B.; Basu, T.; Yap, G.P.A.; Kreisel, K. New Route to the Synthesis of Bis{N-(2-Aminoethyl)Salicylaldiminato}chromium(III) Chloride Monohydrate. Spectrochim. Acta A Mol. Biol. Spectr. 2006, 65, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.H.; Bilton, M.S.; Gill, N.S.; Sterns, M. Isomers of Bis[(R)-N-(Aminopropyl)Salicylaldiminato] -Cobalt(III) and -Chromium(III) Cations. Circular Dichroism and X-Ray Structure Analysis. J. Chem. Soc. Chem. Commun. 1976, 936–937. [Google Scholar] [CrossRef]
- Benson, T.; Bilton, M.; Gill, N. Cobalt(III) and Chromium(III) Complexes of N-(2-Aminoethyl)Salicylaldimine: Crystal Structure and Resolution of the Racemates. Aus. J. Chem. 1977, 30, 261. [Google Scholar] [CrossRef]
- Bilton, M.S. (-)486-S-Bis-[(R-N(2-Amino(R)propyl)salicylaldiminato)cobalt(III)]iodide trihydrate, [C20H26N4O2Co]+I- 3H2O. Cryst. Struct. Commun. 1982, 11, 101–108. [Google Scholar]
- Zhu, H.-L.; Liu, W.-J.; Wang, Y.-F.; Wang, D.-Q. Crystal Structure of Bis[N-(2-Aminopropyl)-Salicylaldiminato)]Cobalt(III) Perchlorate, C20H26ClCoN4O6. Z. Kristallogr. NCS 2003, 218, 255–256. [Google Scholar] [CrossRef] [Green Version]
- Cheetham, A.G.; Claridge, T.D.W.; Anderson, H.L. Metal-Driven Ligand Assembly in the Synthesis of Cyclodextrin [2] and [3]Rotaxanes. Org. Biomol. Chem. 2007, 5, 457–462. [Google Scholar] [CrossRef]
- Thakurta, S.; Butcher, R.J.; Pilet, G.; Mitra, S. Synthesis of Octahedral Cobalt(III) Complexes with Mono- and Di-Condensed Schiff Base Ligands: A Template-Directed Approach for the Isolation of a Rare Kind of Mixed-Ligand Complex. J. Mol. Struct. 2009, 929, 112–119. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Larionov, V.A.; Mkrtchyan, A.F.; Khrustalev, V.N.; Nijland, A.; Saghyan, A.S.; Godovikov, I.A.; Peregudov, A.S.; Babievsky, K.K.; Ikonnikov, N.S.; et al. A Novel Type of Catalysts for the Asymmetric C-C Bond Formation Based on Chiral Stereochemically Inert Cationic Co Iii Complexes. Russ. Chem. Bull. 2012, 61, 2252–2260. [Google Scholar] [CrossRef]
- Fondo, M.; Doejo, J.; García-Deibe, A.M.; Ocampo, N.; Sanmartín, J. Carboxylic Decorated Schiff Base Complexes as Metallotectons for Hydrogen Bonded 3D Networks. Polyhedron 2015, 101, 78–85. [Google Scholar] [CrossRef]
- Maloth, S.; Kurapati, S.K.; Pal, S. Synthesis, Structure, and Properties of a Pentanuclear Cobalt(III) Coordination Cluster. J. Coord. Chem. 2015, 68, 1402–1411. [Google Scholar] [CrossRef]
- DiRisio, R.J.; Armstrong, J.E.; Frank, M.A.; Lake, W.R.; McNamara, W.R. Cobalt Schiff-Base Complexes for Electrocatalytic Hydrogen Generation. Dalton Trans. 2017, 46, 10418–10425. [Google Scholar] [CrossRef]
- Ghosh, K.; Dutta, T.; Drew, M.G.B.; Frontera, A.; Chattopadhyay, S. A Combined Experimental and Theoretical Study on an Ionic Cobalt(III/II) Complex with a Schiff Base Ligand. Polyhedron 2020, 182, 114432. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Maiti, M.; Sadhukhan, D.; Thakurta, S.; Zangrando, E.; Pilet, G.; Signorella, S.; Bellú, S.; Mitra, S. Catalytic Efficacy of Copper(II)– and Cobalt(III)–Schiff Base Complexes in Alkene Epoxidation. Bull. Chem. Soc. Jpn. 2014, 87, 724–732. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; He, Z.-Z.; Liu, Z.; Wang, E. Two New Homometallic Coordination Polymers Based on a Carboxylate-Functionalized Salen Ligand. Inorg. Chem. Commun. 2015, 55, 88–91. [Google Scholar] [CrossRef]
- Fleck, M.; Karmakar, D.; Ghosh, M.; Ghosh, A.; Saha, R.; Bandyopadhyay, D. Synthetic Aspects, Crystal Structure and Antibacterial Activity of Two New Schiff Base Cobalt(III) Complexes. Polyhedron 2012, 34, 157–162. [Google Scholar] [CrossRef]
- Larionov, V.A.; Markelova, E.P.; Smol’yakov, A.F.; Savel’yeva, T.F.; Maleev, V.I.; Belokon, Y.N. Chiral Octahedral Complexes of Co(iii) as Catalysts for Asymmetric Epoxidation of Chalcones under Phase Transfer Conditions. RSC Adv. 2015, 5, 72764–72771. [Google Scholar] [CrossRef]
- Armstrong, J.E.; Crossland, P.M.; Frank, M.A.; Van Dongen, M.J.; McNamara, W.R. Hydrogen Evolution Catalyzed by a Cobalt Complex Containing an Asymmetric Schiff-Base Ligand. Dalton Trans. 2016, 45, 5430–5433. [Google Scholar] [CrossRef]
- Banerjee, S.; Patra, R.; Ghorai, P.; Brandão, P.; Chowdhury, S.G.; Karmakar, P.; Saha, A. Syntheses, Crystal Structures, DNA Binding, DNA Cleavage and DFT Study of Co(iii) Complexes Involving Azo-Appended Schiff Base Ligands. New J. Chem. 2018, 42, 16571–16582. [Google Scholar] [CrossRef]
- Root, C.A.; Hoeschele, J.D.; Cornman, C.R.; Kampf, J.W.; Pecoraro, V.L. Structural and Spectroscopic Characterization of Dioxovanadium(V) Complexes with Asymmetric Schiff Base Ligands. Inorg. Chem. 1993, 32, 3855–3861. [Google Scholar] [CrossRef]
- Nowicka, B.; Samotus, A.; Szklarzewicz, J.; Heinemann, F.W.; Kisch, H. Oxocyano Complexes of Molybdenum(IV) and Tungsten(IV) with Schiff Base Ligands Derived from Salicylaldehyde and Aliphatic Amines. Crystal Structure of [PPh4]2[Mo(CN)3O(Ensal)]·5.5H2O (Hensal = N-Salicylideneethylenediamine). J. Chem. Soc. Dalton Trans. 1998, 4009–4014. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Organometallic Complexes of Schiff Bases: Recent Progress in Oxidation Catalysis. J. Organomet. Chem. 2016, 822, 173–188. [Google Scholar] [CrossRef]
- Curreli, S.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Facile Isolation of Bisimines Based on 3,3′-Diaminobenzidine: Direct Access to Unsymmetrical Bimetallic Salphen Building Blocks. J. Org. Chem. 2007, 72, 7018–7021. [Google Scholar] [CrossRef] [PubMed]
- Curreli, S.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. A Modular Approach Towards Nonsymmetrical Bis(Metallosalen) Building Blocks. Eur. J. Inorg. Chem. 2008, 2008, 2863–2873. [Google Scholar] [CrossRef]
- Castilla, A.M.; Curreli, S.; Carretero, N.M.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Templated Synthesis and Site-Selective Conversion of Completely Nonsymmetrical Bis-Metallosalphen Complexes. Eur. J. Inorg. Chem. 2009, 2009, 2467–2471. [Google Scholar] [CrossRef]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Autocatalytic Demetalation of a Zn(Salphen) Complex Provoked by Unprotected N-Heterocycles. Dalton Trans. 2008, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, S.J.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Colorimetric Discrimination between Important Alkaloid Nuclei Mediated by a Bis-Salphen Chromophore. Org. Lett. 2008, 10, 3311–3314. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Bella, S.D. Aggregation Properties of Bis(Salicylaldiminato)Zinc(ii) Schiff-Base Complexes and Their Lewis Acidic Character. Dalton Trans. 2012, 41, 387–395. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Phase Transition and Vapochromism in Molecular Assemblies of a Polymorphic Zinc(II) Schiff-Base Complex. Inorg. Chem. 2014, 53, 9771–9777. [Google Scholar] [CrossRef]
- Kleij, A.W.; Kuil, M.; Lutz, M.; Tooke, D.M.; Spek, A.L.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Supramolecular Zinc(II)Salphen Motifs: Reversible Dimerization and Templated Dimeric Structures. Inorg. Chim. Acta 2006, 359, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Tschugaeff, L. Ueber ein neues, empfindliches Reagens auf Nickel. Ber. Dtsch. Chem. Ges. 1905, 38, 2520–2522. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzoni, R.; Roncaglia, F.; Rigamonti, L. When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes. Crystals 2021, 11, 483. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11050483
Mazzoni R, Roncaglia F, Rigamonti L. When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes. Crystals. 2021; 11(5):483. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11050483
Chicago/Turabian StyleMazzoni, Rita, Fabrizio Roncaglia, and Luca Rigamonti. 2021. "When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes" Crystals 11, no. 5: 483. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11050483