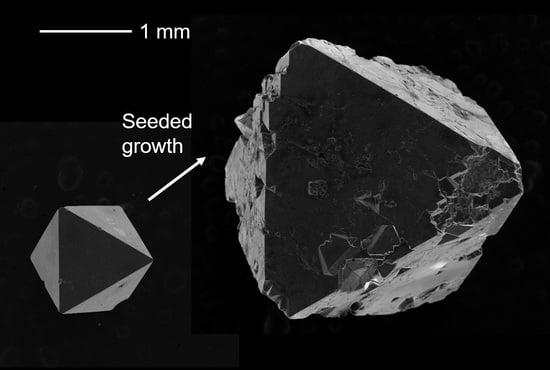
Seeded Growth of Type-II Na24Si136 Clathrate Single Crystals
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nolas, G.S. (Ed.) The Physics and Chemistry of Inorganic Clathrates; Springer: Berlin, Germany, 2014. [Google Scholar]
- Kasper, J.S.; Hagenmuller, P.; Pouchard, M.; Cros, C. Clathrate Structure of Silicon Na8Si46 and NaxSi136 (x < 11). Science 1965, 150, 1713–1714. [Google Scholar] [PubMed]
- Cros, C.; Pouchard, M.; Hagenmuller, P. Sur une nouvelle famille de clathrates minéraux isotypes des hydrates de gaz et de liquides. Interprétation des résultats obtenus. J. Solid State Chem. 1971, 2, 570–581. [Google Scholar] [CrossRef]
- Reny, E.; Gravereau, P.; Cros, C.; Pouchard, M. Structural characterisations of the NaxSi136 and Na8Si46 silicon clathrates using the Rietveld method. J. Mater. Chem. 1998, 8, 2839–2844. [Google Scholar] [CrossRef]
- Ramachandran, G.K.; Dong, J.; Diefenbacher, J.; Gryko, J.; Marzke, R.F.; Sankey, O.F.; McMillan, P.F. Synthesis and X-ray Characterization of Silicon Clathrates. J. Solid State Chem. 1999, 145, 716–730. [Google Scholar] [CrossRef]
- Stefanoski, S.; Malliakas, C.D.; Kanatzidis, M.G.; Nolas, G.S. Synthesis and Structural Characterization of NaxSi136 (0 < x ≤ 24) Single Crystals and Low-Temperature Transport of Polycrystalline Specimens. Inorg. Chem. 2012, 51, 8686–8692. [Google Scholar] [PubMed]
- Mott, N.F. Properties of compounds of type NaxSi46 and NaxSi136. J. Solid State Chem. 1973, 6, 348–351. [Google Scholar] [CrossRef]
- Adams, G.B.; O’Keeffe, M.; Demkov, A.A.; Sankey, O.F.; Huang, Y.-M. Wide-band-gap Si in open fourfold-coordinated clathrate structures. Phys. Rev. B 1994, 49, 8048–8053. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, K.; Munetoh, S.; Shintani, A. First-principles study of Si34−xGex clathrates: Direct wide-gap semiconductors in Si-Ge alloys. Phys. Rev. B 2000, 62, 7138–7143. [Google Scholar] [CrossRef]
- Gryko, J.; McMillan, P.F.; Marzke, R.F.; Ramachandran, G.K.; Patton, D.; Deb, S.K.; Sankey, O.F. Low-density framework form of crystalline silicon with a wide optical band gap. Phys. Rev. B 2000, 62, R7707–R7710. [Google Scholar] [CrossRef]
- Beekman, M.; Baitinger, M.; Borrmann, H.; Schnelle, W.; Meier, K.; Nolas, G.S.; Grin, Y. Preparation and Crystal Growth of Na24Si136. J. Am. Chem. Soc. 2009, 131, 9642–9643. [Google Scholar] [CrossRef] [PubMed]
- Stefanoski, S.; Beekman, M.; Wong-Ng, W.; Zavalij, P.; Nolas, G.S. Simple Approach for Selective Crystal Growth of Intermetallic Clathrates. Chem. Mater. 2011, 23, 1491–1495. [Google Scholar] [CrossRef]
- Stefanoski, S.; Martin, J.; Nolas, G.S. Low temperature transport properties and heat capacity of single-crystal Na8Si46. J. Phys. Condens. Matter 2010, 22, 485404. [Google Scholar] [CrossRef] [PubMed]
- Morito, H.; Shimoda, M.; Yamane, H. Single crystal growth of type I Na–Si clathrate by using Na–Sn flux. J. Cryst. Growth 2016, 450, 164–167. [Google Scholar] [CrossRef]
- Morito, H.; Shimoda, M.; Yamane, H.; Fujiwara, K. Crystal Growth Conditions of Types I and II Na–Si Clathrates by Evaporation of Na from a Na–Si–Sn Solution. Cryst. Growth Des. 2017, 18, 351–355. [Google Scholar] [CrossRef]
- Morito, H.; Yamane, H. Double-Helical Silicon Microtubes. Angew. Chem. Int. Ed. 2010, 49, 3638–3641. [Google Scholar] [CrossRef] [PubMed]
- Morito, H.; Yamada, T.; Ikeda, T.; Yamane, H. Na–Si binary phase diagram and solution growth of silicon crystals. J. Alloy. Compd. 2009, 480, 723–726. [Google Scholar] [CrossRef]
- Sangster, J.; Bale, C.W. The Na-Sn (Sodium-Tin) System. J. Phase Equilibria 1998, 19, 76–81. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morito, H.; Yamane, H.; Umetsu, R.Y.; Fujiwara, K. Seeded Growth of Type-II Na24Si136 Clathrate Single Crystals. Crystals 2021, 11, 808. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070808
Morito H, Yamane H, Umetsu RY, Fujiwara K. Seeded Growth of Type-II Na24Si136 Clathrate Single Crystals. Crystals. 2021; 11(7):808. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070808
Chicago/Turabian StyleMorito, Haruhiko, Hisanori Yamane, Rie Y. Umetsu, and Kozo Fujiwara. 2021. "Seeded Growth of Type-II Na24Si136 Clathrate Single Crystals" Crystals 11, no. 7: 808. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070808