Nanomechanical Characterization of Enzyme Induced Carbonate Precipitates
Abstract
:1. Introduction
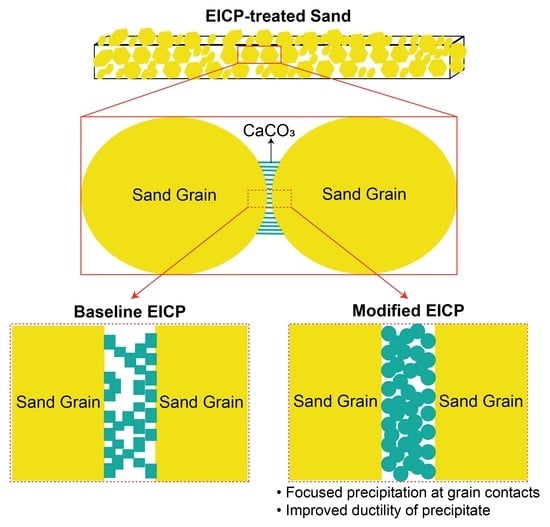
- How are the mechanical and chemical properties of the modified precipitate different from that of the baseline precipitate?
- Apart from favoring the precipitation to occur at interparticle contacts, does the modified EICP treatment contribute to higher UCS also by virtue of the different mechanical characteristics of the precipitate?
2. Materials and Methods
2.1. EICP Solution
2.2. Mineralogical Characterization
2.3. Indentation Testing
3. Results and Discussion
3.1. Mineralogical Characteristics
3.2. Mechanical Properties
3.3. Role of Casein in EICP
3.4. EICP in Porous Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Larsen, J.; Poulsen, M.; Lundgaard, T.; Agerbaek, M. Plugging of fractures in chalk reservoirs by enzyme-induced calcium carbonate precipitation. SPE Prod. Oper. 2008, 23, 478–483. [Google Scholar] [CrossRef]
- Bang, S.S.; Bang, S.; Frutiger, S.; Nehl, L.M.; Comes, B.L. Application of novel biological technique in dust suppression. In Proceedings of the Transportation Research Board 88th Annual Meeting Compendium of Papers, Transportation Research Board, Washington, DC, USA, 11–15 January 2009. [Google Scholar]
- Hamdan, N.; Kavazanjian, E., Jr. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Géotechnique 2016, 66, 546–555. [Google Scholar] [CrossRef]
- Almajed, A.; Khodadadi Tirkolaei, H.; Kavazanjian, E., Jr.; Hamdan, N. Enzyme induced biocementated sand with high strength at low carbonate content. Sci. Rep. 2019, 9, 1135. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Khodadadi Tirkolaei, H.; Kavazanjian, E., Jr. Enhancing the strength of granular material with a modified enzyme-induced carbonate precipitation (EICP) treatment solution. Constr. Build. Mater. 2021, 271, 121529. [Google Scholar] [CrossRef]
- Khodadadi Tirkolaei, H.; Javadi, N.; Krishnan, V.; Hamdan, N.; Kavazanjian, E., Jr. Crude urease extract for biocementation. J. Mater. Civil Eng. 2020, 32, 04020374. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Dominguez-Vera, J.M.; Garcia-Ruiz, J.M. Influence of lysosyme on the precipitation of calcium carbonate: A kinetic and morphologic study. Geochim. Cosmochim. Acta 2003, 67, 1667–1676. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabalah-Amitai, L.; Mayzel, B.; Kauffmann, Y.; Fitch, A.N.; Bloch, L.; Gilbert, P.U.P.A.; Pokroy, B. Vaterite crystals contain two interspersed crystal structures. Science 2013, 340, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. The multiple structures of vaterite. Cryst. Growth Des. 2013, 13, 2247–2251. [Google Scholar] [CrossRef]
- Christy, A.G. A review of the structures of vaterite: The impossible, the possible, and the likely. Cryst. Growth Des. 2017, 17, 3567–3578. [Google Scholar] [CrossRef] [Green Version]
- Holt, C. The Milk Salts and Their Interaction with Casein. In Advanced Dairy Chemistry Volume 3: Lactose, Water, Salts and Vitamins, 2nd ed.; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1997; pp. 233–256. ISBN 978-1-4757-4409-5. [Google Scholar] [CrossRef]
- Zügner, S.; Marquardt, K.; Zimmermann, I. Influence of nanomechanical crystal properties on the comminution process of particulate solids in spiral jet mills. Eur. J. Pharm. Biopharm. 2005, 62, 194–201. [Google Scholar] [CrossRef]
- Merkel, C.; Deuschle, J.; Griesshaber, E.; Enders, S.; Steinhauser, E.; Hochleitner, R.; Brand, U.; Schmahl, W.W. Mechanical properties of modern calcite- (Mergerlia truncata) and phosphate-shelled brachiopods (Discradisca stella and Lingula anatina) determined by nanoindentation. J. Struct. Biol. 2009, 168, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Ganesan, K.; Yang, P.; Kulak, A.N.; Borukhin, S.; Pechook, S.; Ribeiro, L.; Kröger, R.; Eichhorn, S.J.; Armes, S.P.; et al. An artificial biomineral formed by incorporation of copolymer micelles in calcite crystals. Nat. Mater. 2011, 10, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, M.E.; Mangano, L.M.; Peloquin, J.M.; Baker, S.P.; Estroff, L.A. Evaluation of strengthening mechanisms in calcite single crystals from mollusk shells. Acta. Biomater. 2013, 9, 5353–5359. [Google Scholar] [CrossRef]
- Ren, D.; Meyers, M.A.; Zhou, B.; Feng, Q. Comparative study of carp otolith hardness: Lapillus and asteriscus. Mater. Sci. Eng. C 2013, 33, 1876–1881. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Semsarilar, M.; Carloni, J.D.; Cho, K.R.; Kulak, A.N.; Polishchuk, I.; Hendley IV, C.T.; Smeets, P.J.M.; Fielding, L.A.; Pokroy, B.; et al. Structure and properties of nanocomposites formed by the occlusion of block copolymer worms and vesicles within calcite crystals. Adv. Funct. Mater. 2016, 26, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Kabacińska, Z.; Yate, L.; Wencka, M.; Krzyminiewski, R.; Tadyszak, K.; Coy, E. Nanoscale effects of radiation (UV, X-ray, and γ) on calcite surfaces: Implications for its mechanical and physico-chemical properties. J. Phys. Chem. C 2017, 121, 13357–13369. [Google Scholar] [CrossRef]
- Kunitake, M.E.; Baker, S.P.; Estroff, L.A. The effect of magnesium substitution on the hardness of synthetic and biogenic calcite. MRS. Commun. 2012, 2, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning hardness in calcite by incorporation of amino acids. Nat. Mater. 2016, 15, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, C.G.; Ulm, F.-J. Experimental chemo-mechanics of early-age fracture properties of cement paste. Cem. Concr. Res. 2015, 75, 42–52. [Google Scholar] [CrossRef]
- Berthonneau, J.; Hoover, C.G.; Grauby, O.; Baronnet, A.; Pellenq, R.J.-M.; Ulm, F.-J. Crystal-chemistry control of the mechanical properties of 2:1 clay minerals. Appl. Clay Sci. 2017, 143, 387–398. [Google Scholar] [CrossRef]
- Kazembeyki, M.; Bauchy, M.; Hoover, C.G. New insights into the indentation size effect in silicate glasses. J. Non-Cryst. Solids 2019, 521, 119494. [Google Scholar] [CrossRef]
- Qomi, M.J.A.; Krakowiak, K.J.; Bauchy, M.; Stewart, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef]
- Mazzini, V.; Craig, V.S.J. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 8, 7052–7065. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Bentov, S.; Weil, S.; Glazer, L.; Sagi, A.; Berman, A. Stabilization of amorphous calcium carbonate by phosphate rich organic matrix proteins and by single phosphoamino acids. J. Struct. Biol. 2010, 171, 207–215. [Google Scholar] [CrossRef]
- Alvares, K. The role of acidic phosphoproteins in biomineralization. Connect. Tissue Res. 2014, 55, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Cui, Y.J.; Guo, R. Amphiphilic phosphoprotein-controlled formation of amorphous calcium carbonate with hierarchical superstructure. Langmuir 2012, 28, 6097–6105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Y.J.; Mao, H.Y.; Guo, R. Calcium carbonate crystallization in the presence of casein. Cryst. Growth Des. 2012, 12, 4720–4726. [Google Scholar] [CrossRef]
- Krishnan, V.; Khodadadi Tirkolaei, H.; Martin, K.; Hamdan, N.; van Paassen, L.A.; Kavazanjian, E., Jr. Variability in the unconfined compressive strength of EICP-treated ‘standard’ sand. J. Geotech. Geoenviron. Eng. 2021, 147, 06021001. [Google Scholar] [CrossRef]








| Parameter | Baseline Precipitate | Modified Precipitate |
|---|---|---|
| Crystallite size (nm) | 411(5) | 149(2) |
| Microstrain (%) | 0.047(1) | 0.046(3) |
| Calcite unit cell a (Å) | 4.98774(11) | 4.98939(17) |
| Calcite unit cell c (Å) | 17.0659(5) | 17.0770(8) |
| Reference | Description | No. of Indents | Pmax (mN) | H (GPa) | M (GPa) |
|---|---|---|---|---|---|
| Zügner et al., [17] | Geological calcite (ground to diameter 500 μm) | NA | 0.20–1.0 | 2.8–3.4 | 83–88 a |
| Merkel et al., [18] | Inorganic calcite (single crystal, (104) plane) | NA | Note b | 2.0–2.8 | 62–78 c |
| Kim et al., [19] | Synthetic calcite (single crystal, (104) plane) | 10 | Note d | 2.6 ± 0.1 | 89.9 ± 12.4 e |
| Kunitake et al., [20] | Geological calcite (Iceland spar, single crystal) | ||||
| (001) face, 0° azimuth between Berkovich tip and crystal face | 9 | 2.5 | 2.30 ± 0.14 | 76.9 ± 3.1 | |
| (001) face, 60° azimuth between Berkovich tip and crystal face | 9 | 2.5 | 2.46 ± 0.03 | 67.5 ± 1.1 | |
| (104) face, average of azimuths from 0° to 120° | 63 | 2.5 | 2.54 ± 0.07 | 88.1 ± 1.7 | |
| Ren et al., [21] | Pure vaterite crystals in an organic matrix | 31 | 30 | 3.2 ± 0.19 | 57 ± 4.0 |
| Kim et al., [22] | Synthetic calcite (single crystal) | 9 | 10 | 2.45 ± 0.24 | 91.1 ± 5.7 |
| Kabacińska et al., [23] | Single calcite crystal from Wojcieszów quarry, Poland | 36 | 10 | 2.74 ± 0.17 | 70.23 ± 1.35 f |
| Particle No. | No. of Indents a | No. of Outliers | Pmax (mN) | Hardness (H) | Modulus (M) | M, H Correlation (ρ) | ||
|---|---|---|---|---|---|---|---|---|
| Mean (GPa) | SD (GPa) | Mean (GPa) | SD (GPa) | |||||
| 1 | 2 × 13 − 2 | 0 | 2.0 | 3.18 | 0.33 | 59.3 | 5.34 | 0.61 |
| 2 | 4 × 5 | 2 | 2.0 | 3.42 | 0.34 | 56.3 | 3.66 | 0.14 |
| 3 | 5 × 4 | 3 | 2.0 | 3.47 | 0.43 | 62.5 | 4.41 | 0.86 |
| 4 | 3 × 5 − 5 | 1 | 2.0 | 2.98 | 0.52 | 59.2 | 7.89 | 0.96 |
| 5 | 18 | 3 | 2.0 | 2.65 | 0.35 | 36.8 | 5.62 | 0.56 |
| 6 | 10 × 8 | 3 | 2.0 | 3.47 | 0.33 | 60.3 | 5.25 | 0.75 |
| 7 | 9 × 6 | 1 | 2.0 | 3.51 | 0.25 | 50.2 | 5.79 | 0.55 |
| Overall | 213 | 13 | - | 3.37 | 0.41 | 55.8 | 8.67 | 0.56 |
| Particle No. | No. of Indents a | No. of Outliers | Pmax (mN) | Hardness (H) | Modulus (M) | M, H Correlation (ρ) | ||
|---|---|---|---|---|---|---|---|---|
| Mean (GPa) | SD (GPa) | Mean (GPa) | SD (GPa) | |||||
| 1 | 4 × 4 + 4 × 5 | 0 | 2.5 | 2.26 | 0.52 | 42.3 | 6.58 | 0.46 |
| 2 | 5 × 6 | 0 | 2.5 | 3.31 | 0.57 | 54.7 | 2.89 | 0.45 |
| 3 | 4 × 8 | 8 | 2.0 | 3.51 | 0.57 | 58.8 | 5.07 | 0.45 |
| 4 | 8 × 15 | 6 | 2.0 | 2.29 | 0.47 | 47.3 | 6.60 | 0.73 |
| 5 | 8 × 15 | 0 | 2.0 | 2.95 | 0.24 | 50.6 | 2.39 | 0.79 |
| 6 | 9 × 16 | 15 | 2.0 | 3.99 | 0.33 | 61.1 | 2.80 | 0.66 |
| 7 | 10 × 12 | 0 | 2.0 | 2.78 | 0.34 | 53.2 | 3.91 | 0.67 |
| 8 | 12 × 16 | 1 | 2.0 | 2.90 | 0.26 | 50.8 | 3.02 | 0.64 |
| Overall | 764 | 30 | - | 2.99 | 0.65 | 52.3 | 6.39 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, V.; Khodadadi Tirkolaei, H.; Kazembeyki, M.; van Paassen, L.A.; Hoover, C.G.; Seto, J.; Kavazanjian, E., Jr. Nanomechanical Characterization of Enzyme Induced Carbonate Precipitates. Crystals 2022, 12, 995. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12070995
Krishnan V, Khodadadi Tirkolaei H, Kazembeyki M, van Paassen LA, Hoover CG, Seto J, Kavazanjian E Jr. Nanomechanical Characterization of Enzyme Induced Carbonate Precipitates. Crystals. 2022; 12(7):995. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12070995
Chicago/Turabian StyleKrishnan, Vinay, Hamed Khodadadi Tirkolaei, Maryam Kazembeyki, Leon A. van Paassen, Christian G. Hoover, Jong Seto, and Edward Kavazanjian, Jr. 2022. "Nanomechanical Characterization of Enzyme Induced Carbonate Precipitates" Crystals 12, no. 7: 995. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12070995