Crystal Chemistry and Structural Complexity of Natural and Synthetic Uranyl Selenites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurrence
2.2. Single-Crystal X-Ray Diffraction Study
2.3. Coordination of U and Se
2.4. Graphical Representation and Anion Topologies
2.5. Complexity Calculations
3. Results
3.1. Uranyl Selenite Minerals
3.2. Synthetic Uranyl Compounds with Selenite Ions
3.3. Topological Analysis
3.4. Structural and Topological Complexity
4. Discussion
 cm11 rod symmetry group. However, its highest (topological) symmetry is described by the centrosymmetric
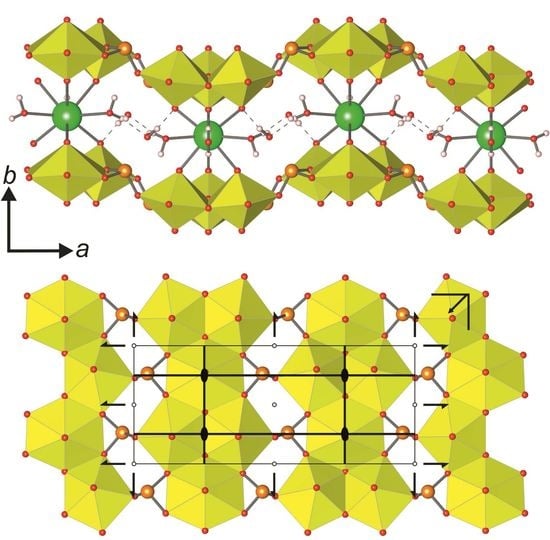
cm11 rod symmetry group. However, its highest (topological) symmetry is described by the centrosymmetric  a2/m11 rod group (Figure 7a). Stacking of chains doubles the complexity contribution of the uranyl selenite block (68.107 bits/cell) into the whole structure, but is still less than the contribution of the Cu-O interstitial block (96.370 bits/cell) and nearly equal to the contribution of the interstitial H-bonding system (64.287 bits/cell; Figure 5 and 6). Alteration of uranyl tetragonal bipyramids by pentagonal ones with the additional H2O molecule in the equatorial plane of Ur preserves the topology, but it doubles the size of the reduced segment of a chain and changes its maximal symmetry to the
a2/m11 rod group (Figure 7a). Stacking of chains doubles the complexity contribution of the uranyl selenite block (68.107 bits/cell) into the whole structure, but is still less than the contribution of the Cu-O interstitial block (96.370 bits/cell) and nearly equal to the contribution of the interstitial H-bonding system (64.287 bits/cell; Figure 5 and 6). Alteration of uranyl tetragonal bipyramids by pentagonal ones with the additional H2O molecule in the equatorial plane of Ur preserves the topology, but it doubles the size of the reduced segment of a chain and changes its maximal symmetry to the  a2/m11 rod group (Figure 7b). The absence of the interstitial substructure makes the topological complexity parameters be equal to those for the whole structure of 8 and 9.
a2/m11 rod group (Figure 7b). The absence of the interstitial substructure makes the topological complexity parameters be equal to those for the whole structure of 8 and 9. a21/m11 and is higher than its real triclinic
a21/m11 and is higher than its real triclinic  -1 symmetry (Figure 7c). In this case, the uranyl selenite substructure (117.207 bits/cell) makes the largest contribution to the complexity of the whole structure. The interstitial complex contributes a slightly lower amount of information (85.926 bits/cell), and even less is accounted for in the H-bonding system (60.842 bits/cell; Figure 5 and 6).
-1 symmetry (Figure 7c). In this case, the uranyl selenite substructure (117.207 bits/cell) makes the largest contribution to the complexity of the whole structure. The interstitial complex contributes a slightly lower amount of information (85.926 bits/cell), and even less is accounted for in the H-bonding system (60.842 bits/cell; Figure 5 and 6).5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierrot, R.; Toussaint, J.; Verbeek, T. La guilleminite, une nouvelle espèce minérale. B. Soc. Fr. Minéral. Cr. 1965, 88, 132–135. [Google Scholar] [CrossRef]
- Cesbron, F.; Bachet, B.; Oosterbosch, R. La demesmaekerite, sélénite hydraté d’uranium, cuivre et plomb. Bulletin B. Soc. Fr. Minéral. Cr. 1965, 88, 422–425. [Google Scholar] [CrossRef]
- Cesbron, F.; Oosterbosch, R.; Pierrot, R. Une nouvelle espèce minérale: La marthozite. Uranyl-sélénite de cuivre hydraté. B. Soc. Fr. Minéral. Cr. 1969, 92, 278–283. [Google Scholar] [CrossRef]
- Cesbron, F.; Pierrot, R.; Verbeek, T. La derriksite, Cu4(UO2)(SeO3)2(OH)6·H2O, une nouvelle espèce minérale. B. Soc. Fr. Minéral. Cr. 1971, 94, 534–537. [Google Scholar]
- Deliens, M.; Piret, P. La haynesite, sélénite hydraté d’uranyle, nouvelle espèce minérale de la Mine Repete, Comté de San Juan, Utah. Can. Mineral. 1991, 29, 561–564. [Google Scholar]
- Vochten, R.; Blaton, N.; Peeters, O.; Deliens, M. Piretite, Ca(UO2)3(SeO3)2(OH)4·4H2O, a new calcium uranyl selenite from Shinkolobwe, Shaba, Zaire. Can. Mineral. 1996, 34, 1317–1322. [Google Scholar]
- Chukanov, N.V.; Pushcharovsky, D.Y.; Pasero, M.; Merlino, S.; Barinova, A.V.; Möckel, S.; Pekov, I.V.; Zadov, A.E.; Dubinchuk, V.T. Larisaite, Na(H3O)(UO2)3(SeO3)2O2·4H2O, a new uranyl selenite mineral from Repete mine, San Juan County, Utah, U.S.A. Eur. J. Mineral. 2004, 16, 367–374. [Google Scholar] [CrossRef]
- Sejkora, J.; Škoda, R.; Pauliš, P. Selenium mineralization of the uranium deposit Zálesí, Rychlebské Hory Mts., Czech Republic. Mineral. Pol. 2006, 28, 196–198. [Google Scholar]
- Gelfort, E. Nutzung der spaltprodtikte nach aufarbeitung ausgedienter brennelemente. Atomwirtsch. Atomtech. 1985, 30, 32–36. [Google Scholar]
- Chen, F.; Burns, P.C.; Ewing, R.C. 79Se: Geochemical and crystallo-chemical retardation mechanisms. J. Nucl. Mater. 1999, 275, 81–88. [Google Scholar] [CrossRef]
- CrysAlisPro Software System; Version 1.171.38.46; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Combinatorial topology of salts of inorganic oxoacids: Zero-, one- and two-dimensional units with corner-sharing between coordination polyhedra. Crystallogr. Rev. 2004, 10, 185–232. [Google Scholar] [CrossRef]
- Burns, P.C.; Miller, M.L.; Ewing, R.C. U6+ minerals and inorganic phases: A comparison and hierarchy of structures. Can. Mineral. 1996, 34, 845–880. [Google Scholar]
- Krivovichev, S.V. Structural Crystallography of Inorganic Oxysalts; Oxford University Press: Oxford, UK, 2008; 303p. [Google Scholar]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals and mineral parageneses: Information and its evolution in the mineral world. In Highlights in Mineralogical Crystallography; Danisi, R., Armbruster, T., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2015; pp. 31–73. [Google Scholar]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar]
- Krivovichev, S.V. Ladders of information: What contributes to the structural complexity in inorganic crystals. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Plasil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. 2019, B75, 39–48. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Krivovichev, S.V.; Charykova, M.V. Selenium minerals: Structural and chemical diversity and complexity. Minerals 2019, 9, 455. [Google Scholar] [CrossRef]
- Cooper, M.A.; Hawthorne, F.C. The crystal structure of guilleminite, a hydrated Ba–U–Se sheet structure. Can. Mineral. 1995, 33, 1103–1109. [Google Scholar]
- Ginderow, D.; Cesbron, F. Structure de la demesmaekerite, Pb2Cu5(SeO3)6(UO2)2(OH)6·2H2O. Acta Crystallogr. 1983, C39, 824–827. [Google Scholar]
- Cooper, M.A.; Hawthorne, F.C. Structure topology and hydrogen bonding in marthozite, Cu2+[(UO2)3(SeO3)2O2](H2O)8, a comparison with guilleminite, Ba[(UO2)3(SeO3)2O2](H2O)3. Can. Mineral. 2001, 39, 797–807. [Google Scholar] [CrossRef]
- Ginderow, D.; Cesbron, F. Structure da la derriksite, Cu4(UO2)(SeO3)2(OH)6. Acta Crystallogr. 1983, C39, 1605–1607. [Google Scholar]
- Cejka, J.; Sejkora, J.; Deliens, M. To the infrared spectrum of haynesite, a hydrated uranyl selenite, and its comparison with other uranyl selenites. Neues Jahbuch Mineral. Monatschefte 1999, 6, 241–252. [Google Scholar]
- Frost, R.L.; Weier, M.L.; Reddy, B.J.; Cejka, J. A Raman spectroscopic study of the uranyl selenite mineral haysenite. J. Raman Spectrosc. 2006, 37, 816–821. [Google Scholar] [CrossRef]
- Loopstra, B.O.; Brandenburg, N.P. Uranyl selenite and uranyl tellurite. Acta Crystallogr. 1978, B34, 1335–1337. [Google Scholar] [CrossRef]
- Diefenbach, K.; Lin, J.; Cross, J.N.; Dalal, N.S.; Shatruk, M.; Albrecht-Schmitt, T.E. Expansion of the rich structures and magnetic properties of neptunium selenites: Soft ferromagnetism in Np(SeO3)2. Inorg. Chem. 2014, 53, 7154–7159. [Google Scholar] [CrossRef]
- Mistryukov, V.E.; Mikhailov, Y.N. Structural features of the selenite group in uranyl complexes with neutral ligands. Koordinats. Khim. 1983, 9, 97–102. [Google Scholar]
- Koskenlinna, M.; Mutikainen, I.; Leskela, T.; Leskela, M. Low-temperature crystal structures and thermal decomposition of uranyl hydrogen selenite monohydrate, [(UO2)(HSeO3)2](H2O) and diammonium uranyl selenite hemihydrate, [NH4]2[(UO2)(SeO3)2](H2O)0.5. Acta Chem. Scand. 1997, 51, 264–269. [Google Scholar] [CrossRef]
- Almond, P.M.; Peper, S.; Bakker, E.; Albrecht-Schmitt, T.E. Variable dimensionality and new uranium oxide topologies in the alkaline-earth metal uranyl selenites AE[(UO2)(SeO3)2] (AE = Ca, Ba) and Sr[(UO2)(SeO3)2] · 2H2O. J. Solid State Chem. 2002, 168, 358–366. [Google Scholar] [CrossRef]
- Almond, P.M.; Albrecht-Schmitt, T.E. Hydrothermal synthesis and crystal chemistry of the new strontium uranyl selenites, Sr[(UO2)3(SeO3)2O2]⋅4H2O and Sr[UO2(SeO3)2]. Am. Mineral. 2004, 89, 976–980. [Google Scholar] [CrossRef]
- Serezhkina, L.B.; Vologzhanina, A.V.; Marukhnov, A.V.; Pushkin, D.V.; Serezhkin, V.N. Synthesis and crystal structure of Na3(H3O)[UO2(SeO3)2]2·H2O. Crystallogr. Rep. 2009, 54, 852–857. [Google Scholar] [CrossRef]
- Koskenlinna, M.; Valkonen, J. Ammonium uranyl hydrogenselenite selenite. Acta Crystallogr. 1996, 52, 1857–1859. [Google Scholar] [CrossRef]
- Almond, P.; Albrecht-Schmitt, T.E. Hydrothermal syntheses, structures, and properties of the new uranyl selenites Ag2(UO2)(SeO3)2, M[(UO2)(HSeO3)(SeO3)] (M = K, Rb, Cs, Tl), and Pb(UO2)(SeO3)2. Inorg. Chem. 2002, 41, 1177–1183. [Google Scholar] [CrossRef]
- Meredith, N.A.; Polinski, M.J.; Lin, J.; Simonetti, A.; Albrecht-Schmitt, T.E. Incorporation of Neptunium(VI) into a uranyl selenite. Inorg. Chem. 2012, 51, 10480–10482. [Google Scholar] [CrossRef]
- Burns, W.L.; Ibers, J.A. Syntheses and structures of three f-element selenite/hydroselenite compounds. J. Solid State Chem. 2009, 182, 1457–1461. [Google Scholar] [CrossRef]
- Marukhnov, A.V.; Pushkin, D.V.; Peresypkina, E.V.; Virovets, A.V.; Serezhkina, L.B. Synthesis and structure of Na[(UO2)(SeO3)(HSeO3)](H2O)4. Rus. J. Inorg. Chem. 2008, 53, 831–836. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Crystal chemistry of selenates with mineral-like structures: VII. The structure of (H3O)[(UO2)(SeO4)(SeO2OH)] and some structural features of selenite-selenates. Geol. Ore Depos. 2009, 51, 663–667. [Google Scholar] [CrossRef]
- Wylie, E.M.; Burns, P.C. Crystal structures of six new uranyl selenate and selenite compounds and their relationship with uranyl mineral structures. Can. Mineral. 2012, 50, 147–157. [Google Scholar] [CrossRef]
- Trombe, J.C.; Gleizes, A.; Galy, J. Structure of a uranyl diselenite, UO2Se2O5. Acta Crystallogr. 1985, C41, 1571–1573. [Google Scholar] [CrossRef]
- Liu, D.-S.; Huang, G.-S.; Luo, Q.-Y.; Xu, Y.-P.; Li, X.-F. Poly[tetramethylammonium [nitratouranyl-µ3-selenito]]. Acta Crystallogr. 2006, E62, 1584–1585. [Google Scholar]
- Almond, P.M.; Albrecht-Schmitt, T.E. Do secondary and tertiary ammonium cations act as structure-directing agents in the formation of layered uranyl selenites? Inorg. Chem. 2003, 42, 5693–5698. [Google Scholar] [CrossRef]
- Liu, D.S.; Kuang, H.M.; Chen, W.T.; Luo, Q.Y.; Sui, Y. Synthesis, structure, and photoluminescence properties of an organically-templated uranyl selenite. Z. Anorg. Allg. Chem. 2015, 641, 2009–2013. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Kahlenberg, V.; Myasoedov, B.F. Synthesis and crystal structure of the first uranyl Selenite(IV)-Selenate(VI) [C5H14N][(UO2)(SeO4)(SeO2OH)]. Dokl. Phys. Chem. 2005, 403, 124–127. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G. Dehydration-driven evolution of topological complexity in ethylamonium uranyl selenates. J. Solid State Chem. 2017, 247, 105–112. [Google Scholar] [CrossRef]
- Jouffret, L.J.; Wylie, E.M.; Burns, P.C. Influence of the organic species and Oxoanion in the synthesis of two uranyl sulfate hydrates, (H3O)2[(UO2)2(SO4)3(H2O)]·7H2O and (H3O)2[(UO2)2(SO4)3(H2O)]·4H2O, and a uranyl Selenate-Selenite [C5H6N][(UO2)(SeO4)(HSeO3)]. Z. Anorg. Allg. Chem. 2012, 638, 1796–1803. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Krivovichev, S.V.; Burns, P.C.; Tananaev, I.G.; Myasoedov, B.F. Supramolecular templates for the synthesis of new nanostructured uranyl compounds: Crystal structure of [NH3(CH2)9NH3][(UO2)(SeO4)(SeO2OH)](NO3). Radiochemistry 2010, 52, 1–6. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Unprecedented layer topology in the crystal structure of a new organically templated uranyl selenite-selenate. Mendeleev Commun. 2012, 22, 11–12. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kovrugin, V.M.; Tyumentseva, O.S.; Mikhailenko, P.A.; Krivovichev, S.V.; Tananaev, I.G. Topologically and geometrically flexible structural units in seven new organically templated uranyl selenates and selenite–selenates. J. Solid State Chem. 2015, 229, 32–40. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Kahlenberg, V.; Myasoedov, B.F. Synthesis and crystal structure of a new uranyl selenite(IV)-selenate(VI), [C5H14N]4[(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)(HSeO4). Radiochemistry 2006, 48, 217–222. [Google Scholar] [CrossRef]
- Wylie, E.M.; Smith, P.A.; Peruski, K.M.; Smith, J.S.; Dustin, M.K.; Burns, P.C. Effects of ionic liquid media on the cation selectivity of uranyl structural units in five new compounds produced using the ionothermal technique. CrystEngComm 2014, 16, 7236–7243. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Tyshchenko, D.V.; Krivovichev, S.V.; Tananaev, I.G. Crown-ether-templated uranyl selenates: Novel family of mixed organic-inorganic actinide compounds. Mendeleev Commun. 2016, 26, 309–311. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Britvin, S.N.; Krivovichev, S.V.; Tananaev, I.G. Ring opening of azetidine cycle: First examples of 1-azetidinepropanamine molecules as a template in hybrid organic-inorganic compounds. J. Mol. Struct. 2018, 1151, 88–96. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Colmont, M.; Siidra, O.I.; Gurzhiy, V.V.; Krivovichev, S.V.; Mentre, O. Pathways for synthesis of new selenium-containing oxo-compounds: Chemical vapor transport reactions, hydrothermal techniques and evaporation method. J. Cryst. Growth 2017, 457, 307–313. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Colmont, M.; Terryn, C.; Colis, S.; Siidra, O.I.; Krivovichev, S.V.; Mentre, O. pH controlled pathway and systematic hydrothermal phase diagram for elaboration of synthetic lead nickel selenites. Inorg. Chem. 2015, 54, 2425–2434. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Ferguson, R.B. Refinement of the crystal structure of kroehnkite. Acta Crystallogr. 1975, B31, 1753–1755. [Google Scholar] [CrossRef]
- Plášil, J.; Hlousek, J.; Kasatkin, A.V.; Novak, M.; Cejka, J.; Lapcak, L. Svornostite, K2Mg[(UO2)(SO4)2]2∙8H2O, a new uranyl sulfate mineral from Jáchymov, Czech Republic. J. Geosci. 2015, 60, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Kampf, A.R.; Sejkora, J.; Witzke, T.; Plášil, J.; Čejka, J.; Nash, B.P.; Marty, J. Rietveldite, Fe(UO2)(SO4)2(H2O)5, a new uranyl sulfate mineral from Giveaway-Simplot mine (Utah, USA), Willi Agatz mine (Saxony, Germany) and Jáchymov (Czech Republic). J. Geosci. 2017, 62, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Izatulina, A.R.; Krivovichev, S.V.; Tananaev, I.G. Chemically induced polytypic phase transitions in the Mg[(UO2)(TO4)2(H2O)](H2O)4 (T = S, Se) system. Inorg. Chem. 2019, 58, 14760–14768. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Burns, P.C. First sodium uranyl chromate, Na4[(UO2)(CrO4)3]: Synthesis and crystal structure determination. Z. Anorg. Allg. Chem. 2003, 629, 1965–1968. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Crystal chemistry of K uranyl chromates: Crystal structures of K8[(UO2)(CrO4)4](NO3)2, K5[(UO2)(CrO4)3](NO3)(H2O)3, K4[(UO2)3(CrO4)5](H2O)8 and K2[(UO2)2(CrO4)3(H2O)2](H2O)4. Z. Kristallogr. 2003, 218, 725–732. [Google Scholar]
- Krivovichev, S.V.; Burns, P.C. Crystal chemistry of uranyl molybdates. VIII. Crystal structures of Na3Tl3[(UO2)(MoO4)4], Na13Tl3[(UO2)(MoO4)3]4(H2O)5, Na3Tl5[(UO2)(MoO4)3]2(H2O)3 and Na2[(UO2)(MoO4)2](H2O)4. Can. Mineral. 2003, 41, 707–720. [Google Scholar] [CrossRef]
- Grigor’ev, M.S.; Fedoseev, A.M.; Budantseva, N.A.; Yanovskii, A.I.; Struchkov, Y.T.; Krot, N.N. Synthesis, crystal and molecular structure of complex neptunium(V) sulfates (Co(NH3)6)(NpO2(SO4)2)·2H2O and (Co(NH3)6) H8O3(NpO2(SO4)3). Sov. Radiokhem. 1999, 33, 54–60. [Google Scholar]
- Norquist, A.J.; Doran, M.B.; Thomas, P.M.; O’Hare, D. Structural diversity in organically templated sulfates. Dalton Trans. 2003, 1168–1175. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Burns, P.C. Structures and syntheses of four Np5+ sulfate chain structures: Divergence from U6+ crystal chemistry. J. Solid State Chem. 2005, 178, 3455–3462. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Novel type of molecular connectivity in one-dimensional uranyl compounds: [K@(18-crown-6)(H2O)][(UO2)(SeO4)(NO3)], a new potassium uranyl selenate with 18-crown-6 ether. Inorg. Chem. Commun. 2014, 45, 93–96. [Google Scholar] [CrossRef]
- Burns, P.C. A new uranyl phosphate chain in the structure of parsonsite. Am. Mineral. 2000, 85, 801–805. [Google Scholar] [CrossRef]
- Locock, A.J.; Burns, P.C.; Flynn, T.M. The role of water in the structures of synthetic hallimondite, Pb2[(UO2)(AsO4)]2(H2O)n and synthetic parsonsite, Pb2[(UO2)(PO4)2](H2O)n, 0 ≤ n ≤ 0.5. Am. Mineral. 2005, 90, 240–246. [Google Scholar] [CrossRef]
- Mills, S.J.; Birch, W.D.; Kolitsch, U.; Mumme, W.G.; Grey, I.E. Lakebogaite, CaNaFe23+H(UO2)2(PO4)4(OH)2(H2O)8, a new uranyl phosphate with a unique crystal structure from Victoria, Australia. Am. Mineral. 2008, 93, 691–697. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Finch, R.; Burns, P.C. Crystal chemistry of uranyl molybdates. V. Topologically different uranyl molybdate sheets in structures of Na2[(UO2)(MoO4)2] and K2[(UO2)(MoO4)2](H2O). Can. Mineral. 2002, 40, 193–200. [Google Scholar] [CrossRef]
- Grigor’ev, M.S.; Charushnikova, I.A.; Fedoseev, A.M.; Budantseva, N.A.; Yanovskii, A.I.; Struchkov, Y.T. Crystal and molecular structure of neptunium(V) complex molybdate K3NpO2(MoO4)2. Sov. Radiokhem. 1992, 34, 7–12. [Google Scholar]
- Krivovichev, S.V.; Kahlenberg, V. Structural diversity of sheets in Rb uranyl selenates: Synthesis and crystal structures of Rb2[(UO2)(SeO4)2(H2O)](H2O), Rb2[(UO2)2(SeO4)3(H2O)2](H2O)4, Rb4[(UO2)3(SeO4)5(H2O)]. Z. Anorg. Allg. Chem. 2005, 631, 739–744. [Google Scholar] [CrossRef]
- Lussier, A.J.; Lopez, R.A.K.; Burns, P.C. A revised and expanded structure hierarchy of natural and synthetic hexavalent uranium compounds. Can. Mineral. 2016, 54, 177–283. [Google Scholar] [CrossRef]
- Christ, C.L.; Clark, J.R.; Evans, H.T., Jr. Crystal structure of rutherfordine, UO2CO3. Science 1955, 121, 472–473. [Google Scholar] [CrossRef]
- Finch, R.J.; Cooper, M.A.; Hawthorne, F.C.; Ewing, R.C. Refinement of the crystal structure of rutherfordine. Can. Mineral. 1999, 37, 929–938. [Google Scholar]
- Demartin, F.; Diella, V.; Donzelli, S.; Gramaccioli, C.M.; Pilati, T. The importance of accurate crystal structure determination of uranium minerals. I. Phosphuranylite KCa(H3O)3(UO2)7(PO4)4O4·8H2O. Acta Crystallogr. 1991, B47, 439–446. [Google Scholar] [CrossRef]
- Shvareva, T.Y.; Albrecht-Schmitt, T.E. General route to three-dimensional framework uranyl transition metal phosphates with atypical structural motifs: The case examples of Cs2{(UO2)4[Co(H2O)2]2(HPO4)(PO4)4} and Cs3+x[(UO2)3CuH4−x(PO4)5]·H2O. Inorg. Chem. 2006, 45, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Plášil, J.; Hauser, J.; Petříček, V.; Meisser, N.; Mills, S.J.; Škoda, R.; Fejfarová, K.; Čejka, J.; Sejkora, J.; Hloušek, J.; et al. Crystal structure and formula revision of deliensite, Fe[(UO2)2(SO4)2(OH)2](H2O)7. Mineral. Mag. 2012, 76, 2837–2860. [Google Scholar] [CrossRef]
- Kampf, A.R.; Kasatkin, A.V.; Čejka, J.; Marty, J. Plášilite, Na(UO2)(SO4)(OH)·2H2O, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. J. Geosci. 2015, 60, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Guesdon, A.; Chardon, J.; Provost, J.; Raveau, B. A copper uranyl monophosphate built up from CuO2 infinity chains: Cu2UO2(PO4)2. J. Solid State Chem. 2002, 165, 89–93. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Synthesis and crystal structure of Li2[(UO2)(MoO4)2], a uranyl molybdate with chains of corner-sharing uranyl square bipyramids and MoO4 tetrahedra. Solid State Sci. 2003, 5, 481–485. [Google Scholar] [CrossRef]
- Almond, P.M.; Albrecht-Schmitt, T.E. Expanding the remarkable structural diversity of uranyl tellurites: Hydrothermal preparation and structures of KUO2Te2O5(OH), Tl3{(UO2)2Te2O5(OH)(Te2O6)}·2H2O, β-Tl2(UO2(TeO3))2, and Sr3((UO2)(TeO3))2(TeO3)2. Inorg. Chem. 2002, 41, 5495–5501. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G. Mineral systems and the thermodynamics of selenites and selenates in the oxidation zone of sulfide ores—A review. Mineral. Petrol. 2017, 111, 121–134. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V.; Vishnevsky, A.V. The thermodynamics of selenium minerals in near-surface environments. Minerals 2017, 7, 188. [Google Scholar] [CrossRef] [Green Version]








| No. | Formula/Mineral Name | Topology | Sp. Gr. | a, Å/α, ° | b, Å/β, ° | c, Å/γ, ° | Reference |
|---|---|---|---|---|---|---|---|
| Chains | |||||||
| 1 | Cu4[(UO2)(SeO3)2](OH)6 derriksite | cc1–1:2–1 | Pn21m | 5.570(2)/90 | 19.088(8)/90 | 5.965(2)/90 | [2] |
| 2 | Pb2Cu5[(UO2)2(SeO3)6(OH)6](H2O)2 demesmaekerite | cc1–1:3–2 | P-1 | 11.9663(9)/89.891(8) | 10.0615(14)/100.341(11) | 5.6318(8)/91.339(9) | This work, [4] |
| Layers with edge-linkage | |||||||
| 3 | Cu[(UO2)3(SeO3)2O2](H2O)8 marthozite | 61524232 | Pbn21 | 6.9879(4)/90 | 16.454(1)/90 | 17.223(1)/90 | [17] |
| 4 | Ba[(UO2)3(SeO3)2O2](H2O)4 guilleminite | Pmn21 | 16.762(1)/90 | 7.2522(5)/90 | 7.0629(4)/90 | This work, [18] | |
| 5 | Na(H3O)[(UO2)3(SeO3)2O2](H2O)4 larisaite | P11m | 6.9806(9)/90 | 7.646(1)/90 | 17.249(2)/90.039(4) | [19] | |
| 6 | [(UO2)3(SeO3)2(OH)2](H2O)5 haynesite | Pnc2 or Pncm | 6.935/90 | 8.025/90 | 17.430/90 | [21] | |
| 7 | Ca[(UO2)3(SeO3)2(OH)4](H2O)4 piretite | Pmn21 or Pmnm | 7.010(3)/90 | 17.135(7)/90 | 17.606(4)/90 | [22] |
| No. | Formula | Topology | Sp. Gr. | a, Å/α, ° | b, Å/β, ° | c, Å/γ, ° | Reference |
|---|---|---|---|---|---|---|---|
| Chains | |||||||
| 8 | [(UO2)(HSeO3)2(H2O)] | cc1–1:2–1 | A2/a | 6.354(1)/90 | 12.578(2)/82.35(1) | 9.972(2)/90 | [35] |
| 9 | [(UO2)(HSeO3)2](H2O) | C2/c | 9.924(5)/90 | 12.546(5)/98.090(5) | 6.324(5)/90 | [36] | |
| 10 | Ca[(UO2)(SeO3)2] | cc1–1:2–14 | P−1 | 5.5502(6)/104.055(2) | 6.6415(7)/93.342(2) | 11.013(1)/110.589(2) | [37] |
| 11 | Sr[(UO2)(SeO3)2] | P−1 | 5.6722(4)/104.698(1) | 6.7627(5)/93.708(1) | 11.2622(8)/109.489(1) | [38] | |
| 12 | Sr[(UO2)(SeO3)2](H2O)2 | cc1–1:2–15 | P−1 | 7.0545(5)/106.995(1) | 7.4656(5)/108.028(1) | 10.0484(6)/98.875(1) | [37] |
| 13 | Na3[H3O][(UO2)(SeO3)2]2(H2O) | P−1 | 9.543(6)/66.69(2) | 9.602(7)/84.10(2) | 11.742(8)/63.69(1) | [39] | |
| Layers with corner-linkage | |||||||
| 14 | [NH4]2[(UO2)(SeO3)2](H2O)0.5 | cc2–1:2–4 | P21/c | 7.193(5)/90 | 10.368(5)/91.470(5) | 13.823(5)/90 | [36] |
| 15 | [NH4][(UO2)(SeO3)(HSeO3)] | P21/n | 8.348(2)/90 | 10.326(2)/97.06(2) | 9.929(2)/90 | [40] | |
| 16 | K[(UO2)(HSeO3)(SeO3)] | P21/n | 8.4164(4)/90 | 10.1435(5)/97.556(1) | 9.6913(5)/90 | [41] | |
| 17 | Rb[(UO2)(HSeO3)(SeO3)] | P21/n | 8.4167(5)/90 | 10.2581(6)/96.825(1) | 9.8542(5)/90 | [41] | |
| 18 | Cs[(UO2)(HSeO3)(SeO3)] | P21/c | 13.8529(7)/90 | 10.6153(6)/101.094(1) | 12.5921(7)/90 | [41,42] | |
| 19 | Cs[((U,Np)O2)(HSeO3)(SeO3)] | P21/n | 8.4966(2)/90 | 10.3910(3)/93.693(1) | 10.2087(3)/90 | [42] | |
| 20 | Tl[(UO2)(HSeO3)(SeO3)] | P21/n | 8.364(3)/90 | 10.346(4)/97.269(8) | 9.834(4)/90 | [41] | |
| 21 | Cs[(UO2)(SeO3)(HSeO3)](H2O)3 | P21/n | 8.673(2)/90 | 10.452(3)/105.147(4) | 13.235(4)/90 | [43] | |
| 22 | Na[(UO2)(SeO3)(HSeO3)](H2O)4 | P21/n | 8.8032(5)/90 | 10.4610(7)/105.054(2) | 13.1312(7)/90 | [44] | |
| 23 | [H3O][(UO2)(SeO4)(HSeO3)] | P21/n | 8.668(2)/90 | 10.655(2)/97.88(2) | 9.846(2)/90 | [45] | |
| 24 | Ag2[(UO2)(SeO3)2] | cc2–1:2–5 | P21/n | 5.8555(6)/90 | 6.5051(7)/96.796(2) | 21.164(2)/90 | [41] |
| Layers with edge-linkage | |||||||
| 25 | Pb[(UO2)(SeO3)2] | cc2–1:2–19 | Pmc21 | 11.9911(7)/90 | 5.7814(3)/90 | 11.2525(6)/90 | [41] |
| 26 | Ba[(UO2)(SeO3)2] | cc2–1:2–21 | P21/c | 7.3067(6)/90 | 8.1239(7)/100.375(2) | 13.651(1)/90 | [37] |
| 27 | [(UO2)(SeO3)] | 6132 | P21/m | 5.408(2)/90 | 9.278(1)/93.45(10) | 4.254(1)/90 | [33] |
| 28 | Sr[(UO2)3(SeO3)2O2](H2O)4 | 61524232 | C2/m | 17.014(2)/90 | 7.0637(7)/100.544(2) | 7.1084(7)/90 | [38] |
| 29 | Li2[(UO2)3(SeO3)2O2](H2O)6 | P21/c | 7.5213(9)/90 | 7.0071(8)/98.834(2) | 17.328(2)/90 | [46] | |
| 30 | Cs2[(UO2)4(SeO3)5](H2O)2 | 61534635 | P21/n | 10.913(3)/90 | 12.427(3)/90.393(3) | 18.448(4)/90 | [46] |
| 31 | Cs2[(UO2)7(SeO4)2(SeO3)2(OH)4O2](H2O)5 | 61564636 | P21/m | 9.1381(3)/90 | 15.0098(5)/91.171(1) | 15.1732(5)/90 | [46] |
| 32 | UO2Se2O5 | 815238 | P−1 | 9.405(2)/93.01(3) | 11.574(2)/93.66(3) | 6.698(2)/109.69(1) | [47] |
| Organically templated | |||||||
| 33 | [C4H12N][(UO2)(SeO3)(NO3)] | cc1–1:2–12 | C2/m | 21.888(3)/90 | 6.950(1)/97.618(3) | 8.350(1)/90 | [48] |
| 34 | [C6H14N2]0.5[(UO2)(HSeO3)(SeO3)](H2O)0.5(CH3CO2H)0.5 | cc2–1:2–4 | Pnma | 13.086(1)/90 | 17.555(1)/90 | 10.5984(7)/90 | [49] |
| 35 | [C4H12N2]0.5[(UO2)(HSeO3)(SeO3)] | P21/c | 10.9378(5)/90 | 8.6903(4)/90.3040(8) | 9.9913(5)/90 | [49] | |
| 36 | [(C2H8N2)H2][(UO2)(SeO3)(HSeO3)](NO3)(H2O)0.5 | Pbca | 13.170(3)/90 | 11.055(2)/90 | 18.009(4)/90 | [50] | |
| 37 | [C5H14N][(UO2)(SeO4)(HSeO3)] | P21/n | 11.553(2)/90 | 10.645(2)/108.05(2) | 12.138(2)/90 | [51] | |
| 38 | [C2H8N][(UO2)(SeO4)(HSeO3)] | P21/n | 8.475(3)/90 | 12.264(2)/95.23(3) | 10.404(3)/90 | [52] | |
| 39 | [C5H6N][(UO2)(SeO4)(HSeO3)] | P21/n | 8.993(3)/90 | 13.399(5)/108.230(4) | 10.640(4)/90 | [53] | |
| 40 | [C9H24N2][(UO2)(SeO4)(HSeO3)](NO3) | P−1 | 10.748(1)/109.960(1) | 13.885(1)/103.212(2) | 14.636(1)/90.409(1) | [54] | |
| 41 | [C2H8N][(H5O2)(H2O)][(UO2)2(SeO4)3(H2SeO3)](H2O) | cc2–1:2–14 | P21/n | 14.798(1)/90 | 10.024(1)/111.628(1) | 16.418(1)/90 | [55] |
| 42 | [C4H15N3][H3O]0.5[(UO2)2(SeO4)2.93(SeO3)0.07(H2O)](NO3)0.5 | cc2–2:3–4 | P21/c | 11.1679(4)/90 | 10.9040(4)/98.019(1) | 17.991(1)/90 | [56] |
| 43 | [C5H14N]4[(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)(HSeO4) | cc2–3:5–3 | P−1 | 11.707(1)/73.90(1) | 14.817(1)/76.22(1) | 16.977(2)/89.36(1) | [57] |
| 44 | [C2H8N]3(C2H7N)[(UO2)3(SeO4)4(HSeO3)(H2O)] | Pnma | 11.659(1)/90 | 14.956(2)/90 | 22.194(2)/90 | [56] | |
| 45 | [C2H8N]2[H3O][(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)0.2 | P21/m | 8.3116(4)/90 | 18.636(1)/97.582(1) | 11.5623(5)/90 | [56] | |
| 46 | [C8H15N2]2[(UO2)4(SeO3)5] | 61534635 | Pnma | 18.860(2)/90 | 18.010(2)/90 | 11.140(1)/90 | [58] |
| No. | Formula | Topology | Complexity Parameters of the Crystal Structure | Structural Complexity of the U-Se Unit | Topological Complexity of the U-Se Unit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp. Gr. | ν | IG | IG,total | Layer or Rod Sym. Gr. | ν | IG | IG,total | Layer or Rod Sym. Gr. | ν | IG | IG,total | |||
| Chains | ||||||||||||||
| 1 | Cu4[(UO2)(SeO3)2](OH)6/derriksite | cc1–1:2–1 | Pn21m | 54 | 4.236 | 228.764 |  cm11 cm11 | 11 | 3.096 | 34.054 |  a2/m11 a2/m11 | 11 | 2.187 | 24.054 |
| 8 | [(UO2)(HSeO3)2(H2O)] | A2/a | 32 | 3.125 | 100.000 |  a12/a1 a12/a1 | 32 | 3.125 | 100.000 |  a12/a1 a12/a1 | 32 | 3.125 | 100.000 | |
| 9 | [(UO2)(HSeO3)2](H2O) | C2/c | ||||||||||||
| 2 | Pb2Cu5[(UO2)2(SeO3)6(OH)6](H2O)2/demesmaekerite | cc1–1:3–2 | P−1 | 55 | 4.800 | 263.975 |  -1 -1 | 30 | 3.907 | 117.207 |  a21/m11 a21/m11 | 30 | 3.374 | 101.207 |
| 33 | [C4H12N][(UO2)(SeO3)(NO3)] | cc1–1:2–12 | C2/m | 56 | 4.236 | 237.212 |  a21/m11 a21/m11 | 22 | 3.096 | 68.108 |  a21/m11 a21/m11 | 22 | 3.096 | 68.108 |
| 10 | Ca[(UO2)(SeO3)2] | cc1–1:2–14 | P−1 | 24 | 3.585 | 86.039 |  -1 -1 | 22 | 3.459 | 76.108 |  -1 -1 | 22 | 3.459 | 76.108 |
| 11 | Sr[(UO2)(SeO3)2] | |||||||||||||
| 12 | Sr[(UO2)(SeO3)2](H2O)2 | cc1–1:2–15 | P−1 | 36 | 4.170 | 150.117 |  -1 -1 | 22 | 3.459 | 76.108 |  -1 -1 | 22 | 3.459 | 76.108 |
| 13 | Na3[H3O][(UO2)(SeO3)2]2(H2O) | 64 | 5.000 | 320.000 | ||||||||||
| Layers with corner-linkage | ||||||||||||||
| 14 | [NH4]2[(UO2)(SeO3)2](H2O)0.5 | cc2–1:2–4 | P21/c | 94 | 4.576 | 430.131 | p21/b | 44 | 3.459 | 152.196 | p21/b | 44 | 3.459 | 152.196 |
| 15 | [NH4][(UO2)(SeO3)(HSeO3)] | P21/n | 68 | 4.087 | 277.947 | p21/b | 48 | 3.585 | 172.080 | 48 | 3.585 | 172.080 | ||
| 16 | K[(UO2)(HSeO3)(SeO3)] | P21/n | 52 | 3.700 | 192.423 | p21/b | 48 | 3.585 | 172.080 | |||||
| 17 | Rb[(UO2)(HSeO3)(SeO3)] | P21/n | 52 | 3.700 | 192.423 | p21/b | 48 | 3.585 | 172.080 | |||||
| 18 | Cs[(UO2)(HSeO3)(SeO3)] | P21/c | 104 | 4.700 | 488.846 | p21 | 48 | 4.585 | 172.080 | |||||
| 19 | Cs[((U,Np)O2)(HSeO3)(SeO3)] | P21/n | 52 | 3.700 | 192.423 | p21/b | 48 | 3.585 | 172.080 | |||||
| 20 | Tl[(UO2)(HSeO3)(SeO3)] | P21/n | 52 | 3.700 | 192.423 | p21/b | 48 | 3.585 | 172.080 | |||||
| 21 | Cs[(UO2)(SeO3)(HSeO3)](H2O)3 | P21/n | 88 | 4.459 | 392.430 | p21/b | 48 | 3.585 | 172.080 | |||||
| 22 | Na[(UO2)(SeO3)(HSeO3)](H2O)4 | P21/n | 100 | 4.644 | 464.386 | p21/b | 48 | 3.585 | 172.080 | |||||
| 34 | [C6H14N2]0.5[(UO2)(HSeO3)(SeO3)](H2O)0.5(CH3CO2H)0.5 | Pnma | 228 | 4.991 | 1137.899 | p21/b | 48 | 3.585 | 172.080 | |||||
| 35 | [C4H12N2]0.5[(UO2)(HSeO3)(SeO3)] | P21/c | 84 | 4.392 | 368.955 | p21/b | 48 | 3.585 | 172.080 | |||||
| 36 | [(C2H8N2)H2][(UO2)(SeO3)(HSeO3)](NO3)(H2O)0.5 | Pbca | 264 | 5.044 | 1331.720 | p21/b | 48 | 3.585 | 172.080 | |||||
| 23 | [H3O][(UO2)(SeO4)(HSeO3)] | P21/n | 68 | 4.087 | 277.947 | p21/b | 52 | 3.700 | 192.423 | 52 | 3.700 | 192.423 | ||
| 37 | [C5H14N][(UO2)(SeO4)(HSeO3)] | P21/n | 132 | 5.044 | 665.860 | |||||||||
| 38 | [C2H8N][(UO2)(SeO4)(HSeO3)] | P21/n | 96 | 4.585 | 440.156 | |||||||||
| 39 | [C5H6N][(UO2)(SeO4)(HSeO3)] | P21/n | 100 | 4.644 | 464.386 | |||||||||
| 40 | [C9H24N2][(UO2)(SeO4)(HSeO3)](NO3) | P-1 | 208 | 6.700 | 1393.691 | p–1 | 52 | 4.700 | 244.423 | |||||
| 24 | Ag2[(UO2)(SeO3)2] | cc2–1:2–5 | P21/n | 52 | 3.700 | 192.423 | p21/b | 44 | 3.459 | 152.215 | p21/b | 44 | 3.459 | 152.215 |
| 41 | [C2H8N][(H5O2)(H2O)][(UO2)2(SeO4)3(H2SeO3)](H2O) | cc2–1:2–14 | P21/n | 204 | 5.672 | 1157.175 | p21/b | 104 | 4.755 | 513.528 | p21/b | 104 | 4.755 | 513.528 |
| 42 | [C4H15N3][H3O]0.5[(UO2)2(SeO4)2.93(SeO3)0.07(H2O)](NO3)0.5 | cc2–2:3–4 | P21/c | 212 | 5.728 | 1214.319 | p21 | 48 | 4.585 | 220.078 | p21 | 48 | 4.585 | 220.078 |
| 43 | [C5H14N]4[(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)(HSeO4) | cc2–3:5–3 | P−1 | 258 | 7.011 | 1808.897 | p–1 | 74 | 5.209 | 385.500 | p21/m | 74 | 4.399 | 325.500 |
| 44 | [C2H8N]3(C2H7N)[(UO2)3(SeO4)4(HSeO3)(H2O)] | Pnma | 364 | 5.629 | 2048.837 | p21/m | 74 | 4.399 | 325.500 | |||||
| 45 | [C2H8N]2[H3O][(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)0.2 | P21/m | 134 | 5.200 | 696.856 | |||||||||
| Layers with Edge-Linkage | ||||||||||||||
| 25 | Pb[(UO2)(SeO3)2] | cc2–1:2–19 | Pmc21 | 48 | 3.835 | 184.078 | p21ma | 44 | 3.641 | 160.215 | p21ma | 44 | 3.641 | 160.215 |
| 26 | Ba[(UO2)(SeO3)2] | cc2–1:2–21 | P21/c | 48 | 3.585 | 172.078 | p21/a | 44 | 3.459 | 152.215 | p21/a | 44 | 3.459 | 152.215 |
| 27 | [(UO2)(SeO3)] | 6132 | P21/m | 14 | 2.236 | 31.303 | p21/m | 14 | 2.236 | 31.303 | p21/m | 14 | 2.236 | 31.303 |
| 3 | Cu[(UO2)3(SeO3)2O2](H2O)8/marthozite | 61524232 | Pbn21 | 176 | 5.459 | 960.860 | pn | 38 | 4.248 | 161.421 | pmmn | 38 | 3.195 | 121.421 |
| 4 | Ba[(UO2)3(SeO3)2O2](H2O)4/guilleminite | Pmn21 | 70 | 4.386 | 307.050 | p21mn | 38 | 3.511 | 133.421 | |||||
| 5 | Na(H3O)[(UO2)3(SeO3)2O2](H2O)4/larisaite | P11m | 73 | 5.395 | 393.857 | pm | 38 | 4.511 | 171.421 | |||||
| 28 | Sr[(UO2)3(SeO3)2O2](H2O)4 | C2/m | 28 | 3.450 | 96.606 | c2/m | 19 | 3.090 | 58.711 | c2/m | 19 | 3.090 | 58.711 | |
| 29 | Li2[(UO2)3(SeO3)2O2](H2O)6 | P21/c | 78 | 4.311 | 336.261 | p21/a | 38 | 3.301 | 125.421 | |||||
| 30 | Cs2[(UO2)4(SeO3)5](H2O)2 | 61534635 | P21/n | 160 | 5.322 | 851.508 | pn | 64 | 5.000 | 320.000 | p21mn | 64 | 4.250 | 272.000 |
| 46 | [C8H15N2]2[(UO2)4(SeO3)5] | Pnma | 328 | 5.455 | 1789.277 | p21mn | 64 | 4.250 | 272.000 | |||||
| 31 | Cs2[(UO2)7(SeO4)2(SeO3)2(OH)4O2](H2O)5 | 61564636 | P21/m | 132 | 5.226 | 689.860 | p–1 | 49 | 4.635 | 227.121 | p–1 | 49 | 4.635 | 227.121 |
| 32 | UO2Se2O5 | 815238 | P−1 | 40 | 4.322 | 172.877 | p1 | 20 | 4.322 | 86.439 | p2 | 20 | 3.422 | 68.439 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurzhiy, V.V.; Kuporev, I.V.; Kovrugin, V.M.; Murashko, M.N.; Kasatkin, A.V.; Plášil, J. Crystal Chemistry and Structural Complexity of Natural and Synthetic Uranyl Selenites. Crystals 2019, 9, 639. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9120639
Gurzhiy VV, Kuporev IV, Kovrugin VM, Murashko MN, Kasatkin AV, Plášil J. Crystal Chemistry and Structural Complexity of Natural and Synthetic Uranyl Selenites. Crystals. 2019; 9(12):639. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9120639
Chicago/Turabian StyleGurzhiy, Vladislav V., Ivan V. Kuporev, Vadim M. Kovrugin, Mikhail N. Murashko, Anatoly V. Kasatkin, and Jakub Plášil. 2019. "Crystal Chemistry and Structural Complexity of Natural and Synthetic Uranyl Selenites" Crystals 9, no. 12: 639. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9120639