Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review
Abstract
:1. Introduction
2. Vaterite Properties
3. Mechanisms of Spherical Vaterite Formation
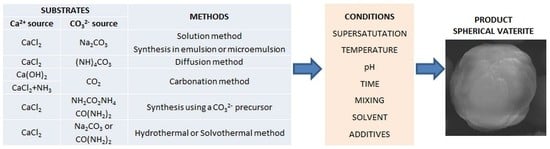
4. Methods of the Synthesis of Vaterite Particles
4.1. Solution Route (L-L)
4.2. Carbonation Route (G-L)
4.3. Diffusion Method
4.4. Synthesis in Emulsions and Microemulsions
4.5. Synthesis Using a Precursor of Carbonate Ions
4.6. Hydrothermal and Solvothermal Methods
5. Factors Influencing Vaterite Formation
5.1. Supersaturation
5.2. Temperature
5.3. pH
5.4. Time
5.5. Mixing
5.6. Additives
6. Summary
Funding
Conflicts of Interest
References
- Friedman, G.M.; Schultz, D.J. Precipitation of vaterite (CaCO3) during oil field drilling. Mineral. Mag. 1994, 58, 401–408. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; MDP Inc.: Chantilly, VA, USA, 2003; Volume 5. [Google Scholar]
- Jones, B. Review of calcium carbonate polymorph precipitation in spring systems. Sediment. Geol. 2017, 353, 64–75. [Google Scholar] [CrossRef]
- Berman, A. Biomineralization of Calcium Carbonate. The Interplay with Biosubstrates. In Metal Ions Life Sciences, Vol. 4; Sigel, A., Freisinger, E., Sigel, R.K.O., Eds.; J. Wiley & Sons Ltd., 2008; pp. 167–205. [Google Scholar]
- Chakoumakos, B.C.; Pracheil, B.M.; Koenigs, R.P.; Bruch, R.M.; Feygenson, M. Empirically testing vaterite structural models using neutron diffraction and thermal analysis. Sci. Rep. 2016, 6, 36799. [Google Scholar] [CrossRef] [Green Version]
- Falini, G.; Fermani, S.; Reggi, M.; Njegić Džakula, B.; Kralj, D. Evidence of structural variability among synthetic and biogenic vaterite. Chem. Commun. 2014, 50, 15370–15373. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.S.; Albarracin, E.J.; Kim, Y.Y.; Ihli, J.; Meldrum, F.C. Confinement stabilises single crystal vaterite rods. Chem. Commun. 2014, 50, 4729–4732. [Google Scholar] [CrossRef] [Green Version]
- Portugal, S.J.; Bowen, J.; Riehl, C. A rare mineral, vaterite, acts as a shock absorber in the eggshell of a communally nesting bird. Ibis 2018, 160, 173–178. [Google Scholar] [CrossRef]
- Wightman, R.; Wallis, S.; Aston, P. Leaf margin organisation and the existence of vaterite-producing hydathodes in the alpine plant Saxifraga scardica. Flora Morphol. Distrib. Funct. Ecol. Plants 2018, 241, 27–34. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Volodkin, D. CaCO3 templated micro-beads and -capsules for bioapplications. Adv. Colloid Interface Sci. 2014, 207, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Liang, T.; Deng, Y.; Qi, X.; Jiang, H.; Wu, Y.; Gao, H. Hierarchical porous calcium carbonate microspheres as drug delivery vector. Prog. Nat. Sci. Mater. Int. 2017, 27, 674–677. [Google Scholar] [CrossRef]
- Svenskaya, Y.I.; Fattah, H.; Zakharevich, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Parakhonskiy, B.V. Ultrasonically assisted fabrication of vaterite submicron-sized carriers. Adv. Powder Technol. 2016, 27, 618–624. [Google Scholar] [CrossRef]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2015, 45, 644–658. [Google Scholar] [CrossRef]
- Vogel, R.; Persson, M.; Feng, C.; Parkin, S.J.; Nieminen, T.A.; Wood, B.; Heckenberg, N.R.; Rubinsztein-Dunlop, H. Synthesis and surface modification of birefringent vaterite microspheres. Langmuir 2009, 25, 11672–11679. [Google Scholar] [CrossRef]
- Parkin, S.J.; Vogel, R.; Persson, M.; Funk, M.; Loke, V.L.Y.; Nieminen, T.A.; Heckenberg, N.R.; Rubinsztein-Dunlop, H. Highly birefringent vaterite microspheres: production, characterization and applicaion for optical micromanipulation. Opt. Express 2009, 17, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Enomae, T.; Isogai, A. Application of Vaterite-Type Calcium Carbonate Prepared by Ultrasound for Ink Jet Paper. J. Imaging Sci. Technol. 2010, 54, 020504-1–020504-6. [Google Scholar] [CrossRef]
- Udrea, I.; Capat, C.; Olaru, E.A.; Isopescu, R.; Mihai, M.; Mateescu, C.D.; Bradu, C. Vaterite synthesis via gas-liquid route under controlled pH conditions. Ind. Eng. Chem. Res. 2012, 51, 8185–8193. [Google Scholar] [CrossRef]
- Nehrke, G.; Van Cappellen, P. Framboidal vaterite aggregates and their transformation into calcite: A morphological study. J. Cryst. Growth 2006, 287, 528–530. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chen, F.B. Polymorphism of CaCO3, precipitated in a constant-composition environment. AIChE J. 1998, 44, 1790–1798. [Google Scholar] [CrossRef]
- Zhan, J.; Lin, H.-P.; Mou, C.-Y. Biomimetic Formation of Porous Single-Crystalline CaCO3 via Nanocrystal Aggregation. Adv. Mater. 2003, 15, 621–623. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Li, F.; Xie, B.; Qian, Y. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. J. Cryst. Growth 2002, 236, 357–362. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Kawano, J.; Shimobayashi, N.; Miyake, A.; Kitamura, M. Precipitation diagram of calcium carbonate polymorphs: Its construction and significance. J. Phys. Condens. Matter 2009, 41, 425102. [Google Scholar] [CrossRef]
- Tas, A.C. Monodisperse calcium carbonate microtablets forming at 70°C in prerefrigerated CaCl2-Gelatin-Urea solutions. Int. J. Appl. Ceram. Technol. 2009, 6, 53–59. [Google Scholar] [CrossRef]
- Christy, A.G. A Review of the Structures of Vaterite: The Impossible, the Possible, and the Likely. Cryst. Growth Des. 2017, 17, 3567–3578. [Google Scholar] [CrossRef]
- Burgess, K.M.N.; Bryce, D.L. On the crystal structure of the vaterite polymorph of CaCO3: A calcium-43 solid-state NMR and computational assessment. Solid State Nucl. Magn. Reson. 2015, 65, 75–83. [Google Scholar] [CrossRef]
- Plummer, N.L.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Ševčík, R.; Šašek, P.; Viani, A. Physical and nanomechanical properties of the synthetic anhydrous crystalline CaCO3 polymorphs: vaterite, aragonite and calcite. J. Mater. Sci. 2018, 53, 4022–4033. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. Spontaneous precipitation of calcium carbonate in the presence of ethanol, isopropanol and diethylene glycol. J. Cryst. Growth 2000, 218, 359–364. [Google Scholar] [CrossRef]
- Barhoum, A.; Ibrahim, H.M.; Hassanein, T.F.; Hill, G.; Reniers, F.; Dufour, T.; Delplancke, M.P.; Van Assche, G.; Rahier, H. Preparation and characterization of ultra-hydrophobic calcium carbonate nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012037. [Google Scholar] [CrossRef] [Green Version]
- Njegić-Džakula, B.; Falini, G.; Brečević, L.; Skoko, Ž.; Kralj, D. Effects of initial supersaturation on spontaneous precipitation of calcium carbonate in the presence of charged poly-l-amino acids. J. Colloid Interface Sci. 2010, 343, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Vdović, N.; Kralj, D. Electrokinetic properties of spontaneously precipitated calcium carbonate polymorphs: The influence of organic substances. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 161, 499–505. [Google Scholar] [CrossRef]
- Perić, J.; Vučak, M.; Krstulović, R.; Brečević, L.; Kralj, D. Phase transformation of calcium carbonate polymorphs. Thermochim. Acta 1996, 277, 175–186. [Google Scholar] [CrossRef]
- Nassrallah-Aboukaïs, N.; Jacquemin, J.; Decarne, C.; Abi-Aad, E.; Lamonier, J. F.; Aboukaïs, A. Transformation of vaterite into calcite in the absence and the presence of copper(II) species. Thermal analysis, IR and EPR study. J. Therm. Anal. Calorim. 2003, 74, 21–27. [Google Scholar] [CrossRef]
- Wolf, G.; Günther, C. Thermophysical investigations of the polymorphous phases of calcium carbonate. J. Therm. Anal. Calorim. 2001, 65, 687–698. [Google Scholar] [CrossRef]
- Maruyama, K.; Kagi, H.; Komatsu, K.; Yoshino, T.; Nakano, S. Pressure-induced phase transitions of vaterite, a metastable phase of CaCO3. J. Raman Spectrosc. 2017, 48, 1449–1453. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Bryce, D.L.; Bultz, E.B.; Aebi, D. Calcium-43 chemical shift tensors as probes of calcium binding environments. Insight into the structure of the vaterite CaCO3 polymorph by 43Ca solid-state NMR spectroscopy. J. Am. Chem. Soc. 2008, 130, 9282–9292. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2011. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.W.; Sham, T.K.; Edén, M.; Hedin, N. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, J.-P.; Lewis, A.E. Classical and Nonclassical Theories of Crystal Growth. In New Perspectives on Mineral Nucleation and Growth; van Driessche, A., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2017; pp. 137–154. [Google Scholar]
- Yang, M.; Jin, X.; Huang, Q. Facile synthesis of vaterite core-shell microspheres. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 374, 102–107. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Xu, J.; Yang, L.; Song, T.; Zhang, P. Synthesis of template-free hollow vaterite CaCO3 microspheres in the H2O/EG system. Mater. Lett. 2013, 104, 28–30. [Google Scholar] [CrossRef]
- Andreassen, J.P.; Flaten, E.M.; Beck, R.; Lewis, A.E. Investigations of spherulitic growth in industrial crystallization. Chem. Eng. Res. Des. 2010, 88, 1163–1168. [Google Scholar] [CrossRef]
- Andreassen, J.-P. Formation mechanism and morphology in precipitation of vaterite-nano-aggregation or crystal growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Mori, Y.; Enomae, T.; Isogai, A. Preparation of pure vaterite by simple mechanical mixing of two aqueous salt solutions. Mater. Sci. Eng. C 2009, 29, 1409–1414. [Google Scholar] [CrossRef]
- Mahtout, L.; Sánchez-Soto, P.J.; Carrasco-Hurtado, B.; Pérez-Villarejo, L.; Takabait, F.; Eliche-Quesada, D. Synthesis of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium. Ceram. Int. 2018, 44, 5291–5296. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Wang, X.; Xiao, B.; Chen, C.; Wu, Y.; Yang, C.; Xu, S. A novel route to prepare the metastable vaterite phase of CaCO3 from CaCl2 ethanol solution and Na2CO3 aqueous solution. Adv. Powder Technol. 2018, 29, 2416–2422. [Google Scholar] [CrossRef]
- Hou, W.; Feng, Q. Morphology and formation mechanism of vaterite particles grown in glycine-containing aqueous solutions. Mater. Sci. Eng. C 2006, 26, 644–647. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X.; Wang, X. Microwave-assisted synthesis of spheroidal vaterite CaCO3 in ethylene glycol-water mixed solvents without surfactants. J. Cryst. Growth 2010, 312, 3191–3197. [Google Scholar] [CrossRef]
- Svenskaya, Y.I.; Fattah, H.; Inozemtseva, O.A.; Ivanova, A.G.; Shtykov, S.N.; Gorin, D.A.; Parakhonskiy, B.V. Key Parameters for Size- and Shape-Controlled Synthesis of Vaterite Particles. Cryst. Growth Des. 2018, 18, 331–337. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Crystallization and transformation of vaterite at controlled pH. J. Cryst. Growth 2006, 289, 269–274. [Google Scholar] [CrossRef]
- Popescu, M.A.; Isopescu, R.; Matei, C.; Fagarasan, G.; Plesua, V. Thermal decomposition of calcium carbonate polymorphs precipitated in the presence of ammonia and alkylamines. Adv. Powder Technol. 2014, 25, 500–507. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kościelska, B.; Karczewski, J.; Gołąbiewska, A. The influence of ammonia and selected amines on the characteristics of calcium carbonate precipitated from calcium chloride solutions via carbonation. Mater. Chem. Phys. 2017, 193, 13–18. [Google Scholar] [CrossRef]
- Prah, J.; Maček, J.; Dražič, G. Precipitation of calcium carbonate from a calcium acetate and ammonium carbamate batch system. J. Cryst. Growth 2011, 324, 229–234. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Zhao, D. Polymorph and morphology of CaCO3 in relation to precipitation conditions in a bubbling system. Chin. J. Chem. Eng. 2017, 25, 1335–1342. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef] [PubMed]
- Massi, M.; Ogden, M.I.; Jones, F. Investigating vaterite phase stabilisation by a tetrazole molecule during calcium carbonate crystallisation. J. Cryst. Growth 2012, 351, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Lyu, S.G.; Park, S.; Sur, G.S. The Synthesis of Vaterite and Physical Properties of PP/CaCO3 Composites. Korean J. Chem. Eng. 1999, 16, 538–542. [Google Scholar] [CrossRef]
- Hirai, T.; Hariguchi, S.; Komasawa, I.; Davey, R.J. Biomimetic Synthesis of Calcium Carbonate Particles in a Pseudovesicular Double Emulsion. Langmuir 2002, 13, 6650–6653. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ahmad, T.; Vaidya, S.; Ahmed, J. Microemulsion route to the synthesis of nanoparticles. Pure Appl. Chem. 2008, 80, 2451–5477. [Google Scholar] [CrossRef]
- Walsh, D.; Lebeau, B.; Mann, S. Morphosynthesis of calcium carbonate (vaterite) microsponges. Adv. Mater. 1999, 11, 324–328. [Google Scholar] [CrossRef]
- Huang, J.H.; Mao, Z.F.; Luo, M.F. Effect of anionic surfactant on vaterite CaCO3. Mater. Res. Bull. 2007, 42, 2184–2191. [Google Scholar] [CrossRef]
- Wang, L.; Sondi, I.; Matijević, E. Preparation of Uniform Needle-Like Aragonite Particles by Homogeneous Precipitation. J. Colloid Interface Sci. 1999, 218, 545–553. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite-calcite-vaterite: A temperature influenced sequential polymorphic transformation of CaCO3in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Kitamura, M. Strategy for control of crystallization of polymorphs. CrystEngComm 2009, 11, 949–964. [Google Scholar] [CrossRef]
- Beck, R.; Andreassen, J.P. The onset of spherulitic growth in crystallization of calcium carbonate. J. Cryst. Growth 2010, 312, 2226–2238. [Google Scholar] [CrossRef]
- Vučak, M.; Perić, J.; Krstulović, R. Precipitation of calcium carbonate in a calcium nitrate and monoethanolamine solution. Powder Technol. 1997, 91, 69–74. [Google Scholar] [CrossRef]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Flaten, E.M.; Seiersten, M.; Andreassen, J.P. Polymorphism and morphology of calcium carbonate precipitated in mixed solvents of ethylene glycol and water. J. Cryst. Growth 2009, 311, 3533–3538. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, W.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Facile synthesis of calcium carbonate with an absolutely pure crystal form using 1-butyl-3-methylimidazolium dodecyl sulfate as the modifier. Colloid Polym. Sci. 2013, 291, 2129–2202. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Kralj, D.; Brecević, L.; Nielsen, A.E. Vaterite growth and dissolution in aqueous solution II. Kinetics of dissolution. J. Cryst. Growth 1994, 143, 269–276. [Google Scholar] [CrossRef]
- Isopescu, R.; Mihai, M.; Capat, C.; Olaru, A.; Mateescu, C.; Dumitrescu, O.; Udrea, I. Modelling of Calcium Carbonate Synthesis by Gas-Liquid Reaction Using CO2 from Flue Gases. Chem. Eng. Trans. 2011, 25, 713–718. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kościelska, B.; Łapiński, M. Precipitation of Spherical Vaterite Particles via Carbonation Route in the Bubble Column and the Gas-Lift Reactor. JOM 2019, 71, 1041–1048. [Google Scholar] [CrossRef]
- Flaten, E.M.; Seiersten, M.; Andreassen, J.P. Growth of the calcium carbonate polymorph vaterite in mixtures of water and ethylene glycol at conditions of gas processing. J. Cryst. Growth 2010, 312, 953–960. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, L.H.; Wang, F.; Wang, Q. Divisive effect of alcohol-water mixed solvents on growth morphology of calcium carbonate crystals. J. Phys. Chem. B 2008, 112, 10668–10674. [Google Scholar] [CrossRef] [PubMed]
- Chuajiw, W.; Takatori, K.; Igarashi, T.; Hara, H.; Fukushima, Y. The influence of aliphatic amines, diamines, and amino acids on the polymorph of calcium carbonate precipitated by the introduction of carbon dioxide gas into calcium hydroxide aqueous suspensions. J. Cryst. Growth 2014, 386, 119–127. [Google Scholar] [CrossRef]
- Wang, C.; Piao, C.; Zhai, X.; Hickman, F.N.; Li, J. Synthesis and character of super-hydrophobic CaCO3 powder in situ. Powder Technol. 2010, 200, 84–86. [Google Scholar] [CrossRef]
- Vucak, M.; Peric, J.; Pons, M.-N. The Influence of Various Admixtures on the Calcium Carbonate Precipitation from a Calcium Nitrate and Monoethanolamine Solution. Chem. Eng. Technol. 1998, 21, 71–75. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Czaplicka, N.; Kościelska, B.; Łapiński, M.; Gębicki, J. Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method. Crystals 2019, 9, 117. [Google Scholar] [CrossRef]
- Štajner, L.; Kontrec, J.; NjegićDžakula, B.; Maltar-Strmečki, N.; Plodinec, M.; Lyons, D.M.; Kralj, D. The effect of different amino acids on spontaneous precipitation of calcium carbonate polymorphs. J. Cryst. Growth 2018, 486, 71–81. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Huang, X.; Wu, G. Biomimetic synthesis of calcium carbonate with different morphologies and polymorphs in the presence of bovine serum albumin and soluble starch. Mater. Sci. Eng. C 2017, 79, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.; Legawiec, K.; Bastrzyk, A.; Fiedot-Toboła, M.; Polowczyk, I. Effect of a lipopeptide biosurfactant on the precipitation of calcium carbonate. Colloids Surfaces B Biointerfaces 2018. [Google Scholar] [CrossRef]




| Properties | Values | Ref. |
|---|---|---|
| Density | 2.54 g/cm3 | [2] |
| 2.65 g/cm3 | [27] | |
| Ksp1 at 25°C | 1.22·10−8 | [29] |
| Ksp for t = 0–90 °C | Ksp = −172.1495–0.077993T + 3074.688/T + 71.595logT | [29] |
| Optical properties | Semitransparent, colorless | [17] |
| Effective birefringence | Δn = 0.06–0.1 | [17] |
| Refractive index | nω = 1.55, nε = 1.65 | [27] |
| αV2 at 25 °C | 35.5·10−6 K−1 | [30] |
| Surface energy | Calculated: 90 mJ/m2; experimental 34–73 mJ/m2 | [31] |
| Analytical technique | Vaterite | Aragonite | Calcite | Ref. |
|---|---|---|---|---|
| FTIR, Wave number, cm−1 | 745 | 710, 713 | 713 | [39] |
| XRD, 2Θ° | 29.5 | 45.9 | 25.0 | [40] |
| Raman, Wave number, cm−1 | 750 | 705 | 711 | [40] |
| 43Ca ssNMR, δiso1, ppm | −3 | −34 | 6 | [41] |
| Additive | Synthesis Method | Influence | Ref. | |||
|---|---|---|---|---|---|---|
| Rate | Stability | Morphology | Size | |||
| Ethanol | L-L, t = 25 °C | + | + | + | [31] | |
| L-L, t = 25–30 °C | + | [81] | ||||
| Iso-propanol | L-L, t = 25 °C | + | + | + | [31] | |
| L-L, t = 25–30 °C | + | [81] | ||||
| Diethylene glycol | L-L, t = 25 °C | + | + | + | [31] | |
| Ethylene glycol | L-L, t = 2–40 °C L-L, t = 25–80 °C L-L, t = 25–50 °C L-L, t = 40, 70 °C Solv., t = 100–150 °C | + | + + | + + | + + | [10] [73] [47] [80] [23] |
| Glycerol | L-L, t = 2–40 °C | + | + | [10] | ||
| Solv., t = 100–150 °C | + | [23] | ||||
| Erythritol | L-L, t = 2–40 °C | + | + | [10] | ||
| 1,2-propanediol | Solv., t = 100–150 °C | + | [23] | |||
| 1,8-diaminooctane | G-S; t = 30 °C | + | [82] | |||
| Glycine | L-L, Diff., ta | + | [52] | |||
| G-S; t = 30 °C | + | [82] | ||||
| 4-aminobutyric acid | G-S; t = 30 °C | + | [82] | |||
| 6-aminohexanoic acid | + | |||||
| Poly-glutamic acid | L-L, t = 25 °C | + | + | [33] | ||
| Poly-aspartic acid | + | + | ||||
| Oleic acid | L-L, t = 30 °C | + | [83] | |||
| EDTMPA | G-L, t = 30, 60 °C | + | + | [84] | ||
| Sucrose | L-L, t = 30 °C | + | [50] | |||
| G-L, t = 22 °C | + | + | [85] | |||
| SDSN | CaCl2+urea; t = 90 °C | + | [66] | |||
| SDBS | + | |||||
| Tween 20 | L-L, ta | + | [49] | |||
| Tween 40 | + | |||||
| Tween 60 | + | |||||
| Tween 80 | + | |||||
| Ionic liquid surfactant | L-L, t = 25 °C | + | + | [47] | ||
| Guar gum | L-L, t = 0, 20, 40 °C | + | [12] | |||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopacka-Łyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals 2019, 9, 223. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040223
Konopacka-Łyskawa D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals. 2019; 9(4):223. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040223
Chicago/Turabian StyleKonopacka-Łyskawa, Donata. 2019. "Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review" Crystals 9, no. 4: 223. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040223