Synthesis, Crystal Structure, Thermal Analysis, and DFT Calculations of Molecular Copper(II) Chloride Complexes with Bitopic Ligand 1,1,2,2-tetrakis(pyrazol-1-yl)ethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumental Characterization Methods
2.2. X-ray Structure Determination
2.3. Computational Chemistry
2.4. Synthesis of Compounds
2.4.1. Synthesis of [Cu2(µ2–Pz4)(DMSO)2Cl4]·4H2O (1)
2.4.2. Synthesis of [Cu2(µ2–Pz4)(DMSO)2Cl4]∙2DMSO (2).
3. Results and Discussion
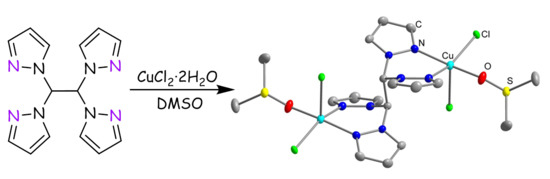
3.1. Synthesis of the Complexes
3.2. Crystal Structures of the Complexes
3.3. Crystal Structure of the Ligand Pz4
3.4. IR Spectroscopy and DFT Calculations
3.5. Thermal and XRD Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kharisov, B.I.; Elizondo Perla, M.; Jiménez-Pérez, V.M.; Kharissova, O.V.; Nájera Martínez, B.; Pérez, N. Recent advances on ditopic ligands. J. Coord. Chem. 2010, 63, 1–25. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Northrop, B.H.; Yang, H.-B.; Stang, P.J. Coordination-driven self-assembly of functionalized supramolecular metallacycles. Chem. Commun. 2008, 5896–5908. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle III, T.; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef]
- Gu, J.; Wen, M.; Cai, Y.; Shi, Z.; Arol, A.S.; Kirillova, M.V.; Kirillov, A.M. Metal-Organic Architectures Assembled from Multifunctional Polycarboxylates: Hydrothermal Self-Assembly, Structures, and Catalytic Activity in Alkane Oxidation. Inorg. Chem. 2019, 58, 2403–2412. [Google Scholar] [CrossRef]
- Gu, J.; Cai, Y.; Wen, M.; Ge, Z.; Kirillov, M.A. New Topologically Unique Metal-Organic Architectures Driven by a Pyridine-Tricarboxylate Building Block. Crystal 2018, 8, 353. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Pettinari, C.; Tăbăcaru, A.; Galli, S. Coordination Polymers and Metal-Organic Frameworks Based on Poly(pyrazole)-containing Ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- Adatoz, E.; Avci, A.K.; Keskin, S. Opportunities and challenges of MOF-based membranes in gas separations. Sep. Purif. Technol. 2015, 152, 207–237. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2015, 307, 361–381. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Z.; Xin, X.; Sun, D. Metal-organic frameworks based luminescent materials for nitroaromatics sensing. CrystEngComm 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Zheng, H.; Xing, L.; Cao, Y.; Che, S. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944. [Google Scholar] [CrossRef]
- Cai, W.; Chu, C.-C.; Liu, G.; Wáng, Y.-X.J. Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar] [CrossRef] [PubMed]
- Zavakhina, M.S.; Khan, I.S.; Barsukova, M.O.; Sapianik, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Chiral guest in a chiral framework: X-ray diffraction study. Russ. Chem. Bull. 2018, 67, 1268–1272. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, Y.; Gu, J.; Fernandes, T.A.; Kirillova, M.V.; Kirillov, A.M. New Copper(II) Coordination Compounds Assembled from Multifunctional Pyridine-Carboxylate Blocks: Synthesis, Structures, and Catalytic Activity in Cycloalkane Oxidation. Molecules 2018, 24, 6. [Google Scholar] [CrossRef]
- Beheshti, A.; Zafarian, H.R.; Abrahams, C.T.; Bruno, G.; Rudbari, H.A. Investigating the effect of anion substitutions on the structure of silver-based coordination polymers. Inorg。 Chim. Acta 2015, 438, 196–202. [Google Scholar] [CrossRef]
- Gardinier, J.R.; Tatlock, H.M.; Hewage, J.S.; Lindeman, S.V. Cyclic versus Polymeric Supramolecular Architectures in Metal Complexes of Dinucleating Ligands: Silver(I) Trifluoromethanesulfonate Complexes of the Isomers of Bis(di(1H-pyrazolyl)methyl)-1,1′-biphenyl. Cryst. Growth Des. 2013, 13, 3864–3877. [Google Scholar] [CrossRef]
- Reger, D.L.; Foley, E.A.; Smith, M.D. Synthesis of a tritopic, third-generation bis(1-pyrazolyl)methane ligand and its silver(I) complex: Unexpected structure with high coordination numbers. Inorg. Chem. Commun. 2010, 13, 568–572. [Google Scholar] [CrossRef]
- Morin, T.J.; Merkel, A.; Lindeman, S.V.; Gardinier, J.R. Breaking the Cycle: Impact of Sterically-Tailored Tetra(pyrazolyl)lutidines on the Self-Assembly of Silver(I) Complexes. Inorg. Chem. 2010, 49, 7992–8002. [Google Scholar] [CrossRef]
- Wang, S.; Zang, H.; Sun, C.; Xu, G.; Wang, X.; Shao, K.; Lan, Y.; Su, Z. Anion-directed genuine meso-helical supramolecular isomers of two 1D Ag(i) complexes based on arene-linked bis(pyrazolyl)methane ligands. CrystEngComm 2010, 12, 3458–3462. [Google Scholar] [CrossRef]
- Reger, D.L.; Watson, R.P.; Smith, M.D. Silver(I) complexes of fixed, polytopic bis(pyrazolyl)methane ligands: Influence of ligand geometry on the formation of discrete metallacycles and coordination polymers. Inorg. Chem. 2006, 45, 10077–10087. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Watson, R.P.; Gardinier, J.R.; Smith, M.D. Impact of Variations in Design of Flexible Bitopic Bis(pyrazolyl)methane Ligands and Counterions on the Structures of Silver(I) Complexes: Dominance of Cyclic Dimeric Architecture. Inorg. Chem. 2004, 43, 6609–6619. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Gardinier, J.R.; Christian Grattan, T.; Smith, M.R.; Smith, M.D. Synthesis of the silver(I) complex of CH2[CH(pz4Et)2]2 containing the unprecedented [Ag(NO3)4]3− anion: A general method for the preparation of 4-(alkyl). New J. Chem. 2003, 27, 1670–1677. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Silaghi-Dumitrescu, I.; Smith, M.D. Influences of Changes in Multitopic Tris(pyrazolyl)methane Ligand Topology on Silver(I) Supramolecular Structures. Inorg. Chem. 2003, 42, 3751–3764. [Google Scholar] [CrossRef] [PubMed]
- Semitut, E.; Komarov, V.; Sukhikh, T.; Filatov, E.; Potapov, A. Synthesis, Crystal Structure and Thermal Stability of 1D Linear Silver(I) Coordination Polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane. Crystals 2016, 6, 138. [Google Scholar] [CrossRef]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis, crystal structure and electrocatalytic activity of discrete and polymeric copper(II) complexes with bitopic bis(pyrazol-1-yl) methane ligands. Inorg. Chem. Commun. 2015, 53, 72–75. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Komarov, V.Y.; Filatov, E.Y.; Kuznetsova, A.S.; Khlebnikov, A.I.; Potapov, A.S. Synthesis and structural characterization of copper(II) coordination polymers with 1,1,2,2-tetra(pyrazol-1-yl)ethane. Inorg. Chem. Commun. 2016, 64, 23–26. [Google Scholar] [CrossRef]
- Dehury, N.; Maity, N.; Tripathy, S.K.; Basset, J.-M.; Patra, S. Dinuclear Tetrapyrazolyl Palladium Complexes Exhibiting Facile Tandem Transfer Hydrogenation/Suzuki Coupling Reaction of Fluoroarylketone. ACS Catal. 2016, 6, 5535–5540. [Google Scholar] [CrossRef]
- Wang, J.-X.; Zhu, Z.-R.; Bai, F.-Y.; Wang, X.-Y.; Zhang, X.-X.; Xing, Y.-H. Molecular design and the optimum synthetic route of the compounds with multi-pyrazole and its derivatives and the potential application in antibacterial agents. Polyhedron 2015, 99, 59–70. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. PowderCell 2.4; Program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns; Federal Institute for Materials Research and Testing: Berlin, Germany, 2000.
- International Centre for Diffraction Data (ICDD). PDF-2 Release; ICDD: Swarthmore, PA, USA, 2014. [Google Scholar]
- PEX2, Version 2.0; SAINT, Version 8.18c; SADABS, Version 2.11; Bruker Advanced X-ray Solutions, Bruker AXS Inc.: Madison, WI, USA, 2000–2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis of new polydentate pyrazolyl-ethene ligands by interaction of 1H-pyrazole and 1,1,2,2-tetrabromoethane in a superbasic medium. J. Heterocycl. Chem. 2011, 48, 645–651. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2[prime or minute]-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalt. Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Martynova, S.A.; Filatov, E.Y.; Korenev, S.V.; Kuratieva, N.V.; Sheludyakova, L.A.; Plusnin, P.E.; Shubin, Y.V.; Slavinskaya, E.M.; Boronin, A.I. Low temperature synthesis of Ru–Cu alloy nanoparticles with the compositions in the miscibility gap. J. Solid State Chem. 2014, 212, 42–47. [Google Scholar] [CrossRef]







| Identification Code | Pz4 | 1 | 2 |
|---|---|---|---|
| Empirical formula | C14H14N8 | C18H34Cl4Cu2N8O6S2 | C22H38Cl4Cu2N8O4S4 |
| Formula weight | 294.33 | 791.53 | 875.72 |
| Temperature/K | 150(2) | 296(2) | 150(2) |
| Crystalsystem | monoclinic | triclinic | monoclinic |
| Spacegroup | C2/c | P-1 | P21/c |
| a/Å | 15.0087(9) | 7.959(3) | 8.7218(4) |
| b/Å | 5.4385(3) | 8.885(3) | 18.2205(7) |
| c/Å | 17.3736(11) | 12.269(5) | 11.9231(5) |
| α/° | 90 | 73.239(16) | 90 |
| β/° | 92.435(2) | 72.615(16) | 107.0408(16) |
| γ/° | 90 | 83.178(18) | 90 |
| Volume/Å3 | 1416.84(15) | 792.3(5) | 1811.58(13) |
| Z | 4 | 1 | 2 |
| ρcalcg/cm3 | 1.380 | 1.659 | 1.605 |
| μ/mm−1 | 0.092 | 1.857 | 1.740 |
| F(000) | 616.0 | 404.0 | 896.0 |
| Crystalsize/mm3 | 0.35 × 0.1 × 0.09 | 0.25 × 0.15 × 0.1 | 0.2 × 0.12 × 0.1 |
| 2Θ range for data collection/° | 4.694–55.148 | 4.792–55.588 | 4.214–52.818 |
| Index ranges | −19 ≤ h ≤ 14, −7 ≤ k ≤ 4, −20 ≤ l ≤ 22 | −9 ≤ h ≤ 10, −11 ≤ k ≤ 11, −14 ≤ l ≤ 15 | −10 ≤ h ≤ 10, −22 ≤ k ≤ 22, −14 ≤ l ≤ 14 |
| Reflections collected | 3290 | 6095 | 24618 |
| Independent reflections | 1628 [Rint = 0.0167, Rsigma = 0.0257] | 3598 [Rint = 0.0391, Rsigma = 0.0579] | 3715 [Rint = 0.0328, Rsigma = 0.0217] |
| Restraints/parameters | 0/100 | 0/189 | 0/203 |
| Goodness-of-fit on F2 | 1.053 | 1.044 | 1.035 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0386, wR2 = 0.0964 | R1 = 0.0424, wR2 = 0.1092 | R1 = 0.0252, wR2 = 0.0556 |
| Final R indexes [all data] | R1 = 0.0494, wR2 = 0.1029 | R1 = 0.0584, wR2 = 0.1153 | R1 = 0.0313, wR2 = 0.0580 |
| Largest diff. peak/hole / e Å−3 | 0.31/−0.23 | 0.70/−0.47 | 0.74/−0.30 |
| Parameter | α | β | τ5 = (β − α)/60 | References |
|---|---|---|---|---|
| [Cu2(Pz4)(DMSO)2Cl4]·4H2O (1) | 129.0 | 171.7 | 0.71 | This paper |
| [Cu2(Pz4)(DMSO)2Cl4]·2DMSO (2) | 153.1 | 168.7 | 0.26 | This paper |
| [Cu2(Pz4)(H2O)2(NO3)4] | 176.0, 178.3 | 168.1, 172.2 | 0.06, 0.17 | [30] |
| [Cu2(Pz4)(H2O)2(NO3)4] | 179.3, 176.1, 177.2, 179.5 | 170.2, 175.4, 173.1, 168.2 | 0.15, 0.01, 0.07, 0.19 | [31] |
| Compound | C1–C1′–Nn1–Nn2 (n = 1, 2) Torsion Angle,° | References |
|---|---|---|
| Pz4 | ±130.1, ±50.9 | This paper |
| [Cu2(Pz4)(DMSO)2Cl4]·4H2O (1) | ±71,8; ±59.5 | This paper |
| [Cu2(Pz4)(DMSO)2Cl4]·2DMSO (2) | ±69,6; ±64.4 | This paper |
| [Cu2(Pz4)(H2O)2(NO3)4] | 60.8; −70.4; 69.1; −61.7 70.4; −61.5; 63.2; −67.0 | [30] |
| [Cu2(Pz4)(H2O)2(NO3)4] | 59.5; −74.9; 67.7; −61.0. | [31] |
| [Cu(Pz4)(NO3)2]n | ±72.2; ±61.5; | [31] |
| [{Cu(Pz4)(H2O)(NO3)2}2]n | ±70.0; ±72.5 | [31] |
| {[Ag(Pz4)(NO3)]DMF}n | ±57.9; ±53.8; ±50.4; ±54.8 | [29] |
| [{Ag(Pz4)(NO3)}n] | ±57.6; ±58.1; ±50.5; ±58.2 | [29] |
| Parameter | Experimental | Calculated | Parameter | Experimental | Calculated |
|---|---|---|---|---|---|
| Pz4 | Complex 1 | ||||
| d(C7-N3), Å | 1.450(1) | 1.452 | d(Cu1-Cl1), Å | 2.436(1) | 2.426 |
| d(N3-N4), Å | 1.355(1) | 1.359 | d(Cu1-Cl2), Å | 2.314(1) | 2.323 |
| d(N4-C4), Å | 1.329(2) | 1.330 | d(Cu1-O1), Å | 1.940(3) | 2.012 |
| d(C4-C5), Å | 1.392(2) | 1.416 | d(Cu1-N1), Å | 2.074(3) | 2.212 |
| d(C5-C6), Å | 1.368(2) | 1.380 | d(Cu1-N3), Å | 2.009(3) | 2.067 |
| d(C6-N3), Å | 1.357(1) | 1.366 | d(S1=O1), Å | 1.533(2) | 1.556 |
| d(C7-C7′), Å | 1.541(1) | 1.558 | d(N3-N4), Å | 1.363(3) | 1.365 |
| d(C7-C7′), Å | 1.551(3) | 1.564 | |||
| φ(O1-Cu1-N3), ° | 171.7(1) | 168.7 | |||
| φ(O1-Cu1-N1), ° | 83.7(1) | 83.4 | |||
| φ(O1-Cu1-Cl2), ° | 94.09(9) | 90.3 | |||
| φ(Cl2-Cu1-Cl1), ° | 116.67(3) | 126.0 | |||
| φ(S1=O1-Cu1), ° | 121.0(2) | 132.5 | |||
| Vibration | Pz4 | [Cu2(Pz4)(DMSO)2Cl4] | |||
|---|---|---|---|---|---|
| calc., cm−1 | exp., cm−1 | calc., cm−1 1 | exp., cm−1 1 | exp., cm−1 2 | |
| νCH (Pz) | 3173 | 3137 | 3139 | 3125 | 3128 |
| νCH (Pz) | 3156 | 3130 | - | - | - |
| νCH (Pz) | 3142 | 3115 | - | - | - |
| νCH (DMSO) | - | - | 3065 | 2987 | 3000 |
| νCH | 3054 | 3018 | 2952 | 2946 | 2943 |
| νCH | 3042 | 2994 | - | - | - |
| νPzasym | 1509 | 1520 | 1509 | 1513 | 1513 |
| δCCH | - | - | 1458 | 1471 | 1469 |
| νPzasym | 1420 | 1437 | 1400 | 1404 | 1405 |
| νPzsym | 1292 | 1311 | 1291 | 1304 | 1302 |
| βCH (Pz) | 1380 | 1391 | 1240 | 1251 | 1254 |
| βCH (Pz) | 1203 | 1216 | 1192 | 1199 | 1200 |
| βCH (Pz) | 1154 | 1172 | 1086 | 1095 | 1095 |
| βCH (Pz) | 1073 | 1092 | 1078 | 1067 | 1068 |
| βCH (Pz) | 1029 | 1053 | 1048 | 1035 | 1032 |
| βCH (Pz) | 947 | 968 | - | - | - |
| δCCH | 1273 | 1293 | - | - | - |
| βCH (Pz) | 899 | 918 | - | - | - |
| γCH | 859 | 890 | - | - | - |
| γCH | 812 | 857 | - | - | - |
| γCH | 740 | 771 | 747 | 769 | 768 |
| γCH | 725 | 754 | - | - | - |
| δNCH | 759 | 783 | 768 | 780 | 789 |
| νSO (DMSO) | - | - | 1003 | 988 | 991 |
| νSO (DMSO) | - | - | 899 | 944 | 944 |
| γCH | 606 | 616 | 603 | 610 | 612 |
| δCCH | 569 | 585 | 548 | 550 | 549 |
| δNCC | 348 | 357 | - | - | - |
| δNCC | 302 | 319 | - | - | - |
| δNCC | 230 | 245 | - | - | - |
| δNCC | 106 | 134 | - | - | - |
| νCu-Cl | - | - | 244 | 254 | 260 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lider, E.; Sukhikh, T.; Smolentsev, A.; Semitut, E.; Filatov, E.; Potapov, A. Synthesis, Crystal Structure, Thermal Analysis, and DFT Calculations of Molecular Copper(II) Chloride Complexes with Bitopic Ligand 1,1,2,2-tetrakis(pyrazol-1-yl)ethane. Crystals 2019, 9, 222. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040222
Lider E, Sukhikh T, Smolentsev A, Semitut E, Filatov E, Potapov A. Synthesis, Crystal Structure, Thermal Analysis, and DFT Calculations of Molecular Copper(II) Chloride Complexes with Bitopic Ligand 1,1,2,2-tetrakis(pyrazol-1-yl)ethane. Crystals. 2019; 9(4):222. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040222
Chicago/Turabian StyleLider, Elizaveta, Taisiya Sukhikh, Anton Smolentsev, Evgeny Semitut, Evgeny Filatov, and Andrei Potapov. 2019. "Synthesis, Crystal Structure, Thermal Analysis, and DFT Calculations of Molecular Copper(II) Chloride Complexes with Bitopic Ligand 1,1,2,2-tetrakis(pyrazol-1-yl)ethane" Crystals 9, no. 4: 222. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040222