Crystalline Modification and Its Effects on Dielectric Breakdown Strength and Space Charge Behavior in Isotactic Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composites and Sample Preparation
2.3. Methods
2.3.1. Field Emission Scanning Electron Microscopy-EDX
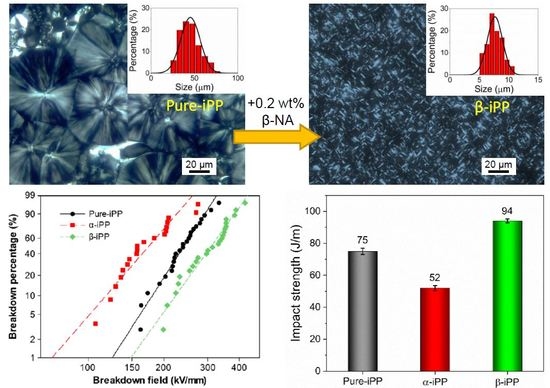
2.3.2. Polarizing Optical Microscopy (POM)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Mechanical Property Measurement
2.3.6. Electrical Property Measurement
3. Results and Discussion
3.1. Crystal Structure Characterization
3.2. Morphology Characterization
3.3. Thermal Characterization
3.4. Mechanical Characterization
3.4.1. Tensile Strength
3.4.2. Flexural Strength
3.4.3. Impact Strength
3.5. Electrical Characterization
3.5.1. DC Conduction Current
3.5.2. DC Breakdown Strength
3.5.3. Space Charge Behavior
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gahleitner, M.; Tranninger, C.; Doshev, P. Heterophasic copolymers of polypropylene: Development, design principles, and future challenges. J. Appl. Polym. Sci. 2013, 130, 3028–3037. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. Structure and properties of isotactic polypropylene. Nuovo Cim. 1960, 15, 40–51. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, W.X.; Zhao, S.C.; Xin, Z.; Shi, Y.Q. Shear-induced β-form polypropylene in long chain branching isotactic polypropylene. Polym. Eng. Sci. 2016, 56, 240–247. [Google Scholar] [CrossRef]
- Meille, S.V.; Bruckner, S.; Porzio, W. γ-Isotactic polypropylene: A structure with nonparallel chain axes. Macromolecules 1990, 23, 4114–4121. [Google Scholar] [CrossRef]
- Kersch, M.; Schmidt, H.W.; Altstadt, V. Influence of different beta-nucleating agents on the morphology of isotactic polypropylene and their toughening effectiveness. Polymer 2016, 98, 320–326. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.B.; Ji, F.F.; Zhang, X.L.; Zheng, G.Q.; Shen, C.Y. Effects of melt structure on shear-induced β-cylindrites of isotactic polypropylene. Polymer 2012, 53, 1791–1800. [Google Scholar] [CrossRef]
- Li, H.; Yan, S. Surface-induced polymer crystallization and the resultant structures and morphologies. Macromolecules 2011, 44, 417–428. [Google Scholar] [CrossRef]
- Pawlak, A.; Piorkowska, E. Crystallization of isotactic polypropylene in a temperature gradient. Colloid. Polym. Sci. 2001, 279, 939–946. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Chua, J.O.; Gryte, C.C. Studies on the alpha and beta forms of isotactic polypropylene by crystallization in a temperature gradient. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 641–656. [Google Scholar] [CrossRef]
- Cao, X.W.; Qiao, Y.H.; Chen, Y.X.; He, G.J.; Lin, H. Critical role of depressurization and effects of saturation conditions in the formation of β-Crystal during isotactic polypropylene foaming with supercritical CO2. Polym. Eng. Sci. 2016, 56, 980–986. [Google Scholar] [CrossRef]
- Zhang, R.H.; Shi, D.A.; Tsui, C.P.; Tang, C.Y.; Tjong, S.C.; Li, R. The formation of β-polypropylene crystals in a compatibilized blend of isotactic polypropylene and polyamide-6. Polym. Eng. Sci. 2011, 51, 403–410. [Google Scholar] [CrossRef]
- Kang, J.; He, J.H.; Chen, Z.F.; Yang, F.; Chen, J.Y.; Cao, Y.; Xiang, M. Effects of β-nucleating agent and crystallization conditions on the crystallization behavior and polymorphic composition of isotactic polypropylene/multi-walled carbon nanotubes composites. Polym. Adv. Technol. 2015, 26, 32–40. [Google Scholar] [CrossRef]
- Krentz, T.; Khani, M.M.; Bell, M.; Benicewicz, B.C.; Nelson, J.K.; Zhao, S.; Hillborg, H.; Schadler, L.S. Morphologically dependent alternating-current and direct-current breakdown strength in silica–polypropylene nanocomposites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhang, L.; Khani, M.M.; Krentz, T.M.; Huang, Y.H.; Zhou, Y.X.; Benicewicz, B.C.; Nelson, J.K.; Schadler, L.S. Suppression of space charge in crosslinked polyethylene filled with poly(stearyl methacrylate)-grafted SiO2 nanoparticles. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Zhang, B.L.; Zhou, Y.X.; Wang, N.H.; Liang, X.D.; Guan, Z.C.; Takada, T. Polarity reversal charging of polypropylene films under DC corona discharge. J. Electrostatics 2005, 63, 657–663. [Google Scholar] [CrossRef]
- Hassinger, I.; Li, X.L.; Zhao, H.; Xu, H.Y.; Huang, Y.H.; Prasad, A.; Schadler, L.; Chen, W.; Catherine, B. Toward the development of a quantitative tool for predicting dispersion of nanocomposites under non-equilibrium processing conditions. J. Mater. Sci. 2016, 51, 4238–4249. [Google Scholar] [CrossRef]
- Bai, H.W.; Wang, Y.; Song, B.; Han, L. Synergistic toughening effects of nucleating agent and ethylene-octene copolymer on polypropylene. J. Appl. Polym. Sci. 2008, 108, 3270–3280. [Google Scholar] [CrossRef]
- Grein, C.; Plummer, C.J.G.; Kausch, H.H.; Germain, Y.; Beguelin, P. Influence of β nucleation on the mechanical properties of isotactic polypropylene and rubber modified isotactic polypropylene. Polymer 2002, 43, 3279–3293. [Google Scholar] [CrossRef]
- Wang, F.F.; Du, H.N.; Liu, H.; Zhang, Y.; Zhang, X.W.; Zhang, J. The synergistic effects of β-nucleating agent and ethylene-octene copolymer on toughening isotactic polypropylene. Polym. Test. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Truong, L.T.; Larsen, A.G.; Roots, J. Morphology, crystalline features, and tensile properties of syndiotactic polypropylene blends. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Inoue, Y.; Onai, T.; Doshu, C.; Takahashi, H.; Uehara, H. Deconvolution analyses of differential scanning calorimetry profiles of β-crystallized polypropylenes with synchronized X-ray measurements. Macromolecules 2007, 40, 2745–2750. [Google Scholar] [CrossRef]
- Riekel, C.; Karger-Cocsis, J. Structural investigation of the phase transformation in the plastic zone of a β-phase isotactic polypropylene by synchrotron radiation microdiffraction. Polymer 1999, 40, 541–545. [Google Scholar] [CrossRef]
- Kotek, J.; Raab, M.; Baldrian, J.; Grellmann, W. The effect of specific β-nucleation on morphology and mechanical behavior of isotactic polypropylene. J. Appl. Polym. Sci. 2002, 86, 1174–1184. [Google Scholar] [CrossRef]
- Aboulfaraj, M.; G’Sell, C.; Ulrich, B.; Dahoun, A. In situ observation of the plastic deformation of polypropylene spherulites under uniaxial tension and simple shear in the scanning electron microscope. Polymer 1995, 36, 731–742. [Google Scholar] [CrossRef]
- Zheng, F.H.; Gu, M.; Dong, J.X.; An, Z.L.; Lei, Q.Q.; Zhang, Y.W. Fast space charge behavior in heat-treated polypropylene films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Dang, B.; Li, Q.; Zhou, Y.; Hu, J.; He, J.L. Suppression of elevated temperature space charge accumulation in polypropylene/elastomer blends by deep traps induced by surface-modified ZnO nanoparticles. Compos. Sci. Technol. 2017, 153, 103–110. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zha, J.W.; Li, W.K.; Wang, S.J.; Dang, Z.M. A remarkable suppression on space charge in isotactic polypropylene by inducing the β-crystal formation. Appl. Phys. Lett. 2015, 107, 112901. [Google Scholar] [CrossRef]
- Zha, J.W.; Yan, H.D.; Li, W.K.; Dang, Z.M. Morphology and crystalline-phase-dependent electrical insulating properties in tailored polypropylene for HVDC cables. Appl. Phys. Lett. 2016, 109, 222902. [Google Scholar] [CrossRef]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Macromol. Chem. Phys. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Lotz, B. α and β phases of isotactic polypropylene: A case of growth kinetics “phase reentrancy” in polymer crystallization. Polymer 1998, 39, 4561–4567. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M. Polypropylene/glass fiber hierarchical composites incorporating inorganic fullerene-like nanoparticles for advanced technological applications. ACS Appl. Mater. Interfaces 2013, 5, 9691–9700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Cheng, W.L.; Jia, D. A study on the heat of fusion of β-polypropylene. Polymer 1999, 40, 1219–1222. [Google Scholar] [CrossRef]
- Galli, P.; Vecellio, G. Technology: Driving force behind innovation and growth of polyolefins. Prog. Polym. Sci. 2001, 26, 1287–1336. [Google Scholar] [CrossRef]
- Peng, H.M.; Wang, B.; Gai, J.G.; Chen, J.Y.; Yang, F.; Cao, Y.; Li, H.L.; Kang, J.; Xiang, M. Investigation on the morphology and tensile behavior of β-nucleated isotactic polypropylene with different stereo-defect distribution. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Li, Y.; Yasuda, M.; Takada, T. Pulsed electroacoustic method for measurement of charge accumulation in solid dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 188–195. [Google Scholar]
- Xu, X.; Li, X.P.; Jin, B.Q.; Sheng, Q.; Wang, T.; Zhang, J. Influence of morphology evolution on the mechanical properties of beta nucleated isotactic polypropylene in presence of polypropylene random copolymer. Polym. Test. 2016, 51, 13–19. [Google Scholar] [CrossRef]
- Somani, R.H.; Hsiao, B.S.; Nogales, A.; Fruitwala, H.; Srinivas, S.; Tsou, A.H. Structure development during shear flow induced crystallization of i-PP: In situ wide-angle X-ray diffraction study. Macromolucules 2001, 34, 5902–5909. [Google Scholar] [CrossRef]
- Jia, C.F.; Liao, X.; Zhu, J.J.; An, Z.; Zhang, Q.W.; Yang, Q.; Li, G.X. Creep-resistant behavior of beta-polypropylene with different crystalline morphologies. RSC Adv. 2016, 6, 30986–30997. [Google Scholar] [CrossRef]
- Varga, J.J. β-Modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. Macromol. Sci. Phys. 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Chen, H.B.; Karger-Kocsis, J.; Wu, J.S.; Varga, J. Fracture toughness of α- and β-phase polypropylene homopolymers and random- and block-copolymers. Polymer 2002, 43, 6505–6514. [Google Scholar] [CrossRef]
- Virtanen, S.; Krentz, T.M.; Nelson, J.K.; Schadler, L.S.; Bell, M.; Benicewicz, B. Dielectric breakdown strength of epoxy bimodal-polymer-brush-grafted core functionalized silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 563–570. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.X.; Teng, C.Y.; Zhang, Y.X.; Cheng, Z.X. Transient Behavior of Space Charge in Heat-Treated Low-Density Polyethylene under Coupled Fields. Sensors Mater. 2017, 29, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Quirke, N. Molecular modeling of electron trapping in polymer insulators. J. Chem. Phys. 2000, 113, 369–376. [Google Scholar] [CrossRef]











| Sample | Crystalline Temperature (°C) | (%) | (%) | (%) | ||
|---|---|---|---|---|---|---|
| Pure-iPP | 118.1 | 42.6 | 0 | 42.6 | 0 | 0 |
| α-iPP | 127.5 | 43.1 | 0 | 43.1 | 0 | 0 |
| β-iPP | 124.6 | 12.0 | 30.3 | 42.3 | 0.72 | 0.81 |
| Voltage Polarity | Sample | Shape Index | Scale Index (kV/mm) | Variation Percentage (%) |
|---|---|---|---|---|
| Negative DC | Pure-iPP | 6.40 | 307.3 | 0 |
| Negative DC | α-iPP | 6.05 | 177.4 | −42.3 |
| Negative DC | β-iPP | 10.49 | 340.1 | +10.7 |
| Positive DC | Pure-iPP | 6.41 | 256.1 | 0 |
| Positive DC | α-iPP | 4.77 | 189.2 | −26.1 |
| Positive DC | β-iPP | 5.72 | 331.0 | +29.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, Y.; Zhou, Y.; Teng, C.; Peng, Z.; Spinella, S. Crystalline Modification and Its Effects on Dielectric Breakdown Strength and Space Charge Behavior in Isotactic Polypropylene. Polymers 2018, 10, 406. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10040406
Zhang L, Zhang Y, Zhou Y, Teng C, Peng Z, Spinella S. Crystalline Modification and Its Effects on Dielectric Breakdown Strength and Space Charge Behavior in Isotactic Polypropylene. Polymers. 2018; 10(4):406. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10040406
Chicago/Turabian StyleZhang, Ling, Yunxiao Zhang, Yuanxiang Zhou, Chenyuan Teng, Zhaowei Peng, and Stephen Spinella. 2018. "Crystalline Modification and Its Effects on Dielectric Breakdown Strength and Space Charge Behavior in Isotactic Polypropylene" Polymers 10, no. 4: 406. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10040406