Influence of the Cross-Link Density on the Rate of Crystallization of Poly(ε-Caprolactone)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cross-Linked PCL
2.2. Equilibrium Swelling Experiments
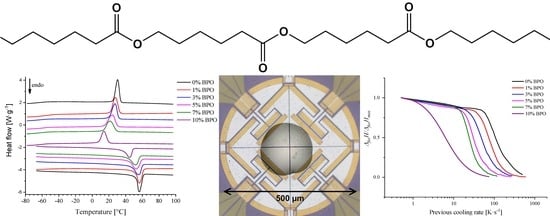
2.3. Conventional DSC Experiments
2.4. FSC Experiments
3. Results
3.1. Equilibrium Swelling Experiments
3.2. Conventional DSC Experiments
3.3. FSC Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Polycaprolactone Market to Reach US$ 214.2 Mn by an End of 2021, Persistence Market Research (PMR) Report 2016. Available online: https://www.persistencemarketresearch.com/mediarelease/polycaprolactone-market.asp (accessed on 1 August 2018).
- Jenkins, M. (Ed.) Biomedical Polymers; Woodhead Publishing in Materials; Woodhead Publ.: Cambridge, UK, 2007; ISBN 978-1-84569-070-0. [Google Scholar]
- Merkli, A.; Tabatabay, C.; Gurny, R.; Heller, J. Biodegradable polymers for the controlled release of ocular drugs. Prog. Polym. Sci. 1998, 23, 563–580. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Vieira, J.C.; Guedes, R.M.; Marques, A.T. Degradation and Viscoelastic Properties of PLA-PCL, PGA-PCL, PDO and PGA Fibres. Mater. Sci. Forum 2010, 636–637, 825–832. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Jin, Y.; Li, M.; Gu, Z. In vitro enzymatic degradation of the cross-linked poly(ε-caprolactone) implants. Polym. Degrad. STable 2015, 112, 10–19. [Google Scholar] [CrossRef]
- Pandini, S.; Passera, S.; Messori, M.; Paderni, K.; Toselli, M.; Gianoncelli, A.; Bontempi, E.; Riccò, T. Two-way reversible shape memory behaviour of crosslinked poly(ε-caprolactone). Polymer 2012, 53, 1915–1924. [Google Scholar] [CrossRef]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic polyesters. I. The degradation of poly(ϵ-caprolactone) in vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of crystalline morphology on the degradation of polycaprolactone in a solution of phosphate buffer and lipase. Polym. Adv. Technol. 2008, 19, 1901–1906. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, B. Crystallization Kinetics in Mixtures of Poly(ε-caprolactone) and Poly(styrene-co-acrylonitrile). Macromolecules 1997, 30, 6223–6229. [Google Scholar] [CrossRef]
- L’Abee, R.; Van Duin, M.; Goossens, H. Crystallization kinetics and crystalline morphology of poly(ε-caprolactone) in blends with grafted rubber particles. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1438–1448. [Google Scholar] [CrossRef] [Green Version]
- Madbouly, S.A. Isothermal crystallization kinetics in binary miscible blend of poly(ε-caprolactone)/tetramethyl polycarbonate. J. Appl. Polym. Sci. 2007, 103, 3307–3315. [Google Scholar] [CrossRef]
- Madbouly, S.A. Nonisothermal Crystallization Kinetics of Miscible Blends of Polycaprolactone and Crosslinked Carboxylated Polyester Resin. J. Macromol. Sci. Part B 2011, 50, 427–443. [Google Scholar] [CrossRef]
- Lai, S.L.; Ramanath, G.; Allen, L.H.; Infante, P.; Ma, Z. High-speed (104 °C/s) scanning microcalorimetry with monolayer sensitivity (J/m 2). Appl. Phys. Lett. 1995, 67, 1229–1231. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. (Eds.) Fast Scanning Calorimetry; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31327-6, 978-3-319-31329-0. [Google Scholar]
- Schick, C.; Androsch, R. Fast Scanning Chip Calorimetry. In Handbook of Thermal Analysis and Calorimetry; Elsevier: New York, NY, USA, 2018; Volume 6, pp. 47–102. ISBN 9780444640628. [Google Scholar]
- McCluskey, P.J.; Vlassak, J.J. Parallel nano-Differential Scanning Calorimetry: A New Device for Combinatorial Analysis of Complex nano-Scale Material Systems. MRS Proc. 2006, 924. [Google Scholar] [CrossRef]
- Lopeandía, A.F.; Cerdó, L. l.; Clavaguera-Mora, M.T.; Arana, L.R.; Jensen, K.F.; Muñoz, F.J.; Rodríguez-Viejo, J. Sensitive power compensated scanning calorimeter for analysis of phase transformations in small samples. Rev. Sci. Instrum. 2005, 76, 065104. [Google Scholar] [CrossRef]
- Mathot, V.; Pyda, M.; Pijpers, T.; Vanden Poel, G.; van de Kerkhof, E.; van Herwaarden, S.; van Herwaarden, F.; Leenaers, A. The Flash DSC 1, a power compensation twin-type, chip-based fast scanning calorimeter (FSC): First findings on polymers. Thermochim. Acta 2011, 522, 36–45. [Google Scholar] [CrossRef]
- Minakov, A.A.; Schick, C. Ultrafast thermal processing and nanocalorimetry at heating and cooling rates up to 1MK/s. Rev. Sci. Instrum. 2007, 78, 073902. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.W.; Abarra, E.N.; Allen, K.; Rooney, P.W.; Messer, M.T.; Watson, S.K.; Hellman, F. Thin film microcalorimeter for heat capacity measurements from 1.5 to 800 K. Rev. Sci. Instrum. 1994, 65, 946–959. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Interplay between the Relaxation of the Glass of Random l/d-Lactide Copolymers and Homogeneous Crystal Nucleation: Evidence for Segregation of Chain Defects. J. Phys. Chem. B 2016, 120, 4522–4528. [Google Scholar] [CrossRef] [PubMed]
- Schawe, J.E.K. Cooling rate dependence of the crystallinity at nonisothermal crystallization of polymers: A phenomenological model. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 91, 239–263. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Madhavi, V.; Lustiger, A.; Androsch, R.; Schick, C. Crystallization of Polyethylene at Large Undercooling. ACS Macro Lett. 2016, 5, 365–370. [Google Scholar] [CrossRef]
- Wurm, A.; Zhuravlev, E.; Eckstein, K.; Jehnichen, D.; Pospiech, D.; Androsch, R.; Wunderlich, B.; Schick, C. Crystallization and Homogeneous Nucleation Kinetics of Poly(ε-caprolactone) (PCL) with Different Molar Masses. Macromolecules 2012, 45, 3816–3828. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly(ε-caprolactone) (PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Wurm, A.; Pötschke, P.; Androsch, R.; Schmelzer, J.W.P.; Schick, C. Kinetics of nucleation and crystallization of poly(ε-caprolactone)—Multiwalled carbon nanotube composites. Eur. Polym. J. 2014, 52, 1–11. [Google Scholar] [CrossRef]
- Hirschl, C.; Biebl-Rydlo, M.; DeBiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Sol. Cells 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briançon, S.; Lantéri, P. Determination of poly(ε-caprolactone) solubility parameters: Application to solvent substitution in a microencapsulation process. Int. J. Pharm. 2010, 383, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-0-8493-0176-6. [Google Scholar]
- Wurm, A.; Merzlyakov, M.; Schick, C. Reversible Melting During Crystallization of Polymers Studied by Temperature Modulated Techniques (TMDSC, TMDMA). J. Therm. Anal. Calorim. 2000, 60, 807–820. [Google Scholar] [CrossRef]
- Skoglund, P. Fransson, ke Continuous cooling and isothermal crystallization of polycaprolactone. J. Appl. Polym. Sci. 1996, 61, 2455–2465. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Gaylord, R.J. A theory of the stress-induced crystallization of crosslinked polymeric networks. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 1827–1837. [Google Scholar] [CrossRef]
- Tosaka, M.; Senoo, K.; Kohjiya, S.; Ikeda, Y. Crystallization of stretched network chains in cross-linked natural rubber. J. Appl. Phys. 2007, 101, 084909. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Wang, Z.; Liang, X.; Hu, Y. Non-isothermal crystallization kinetics of silane crosslinked polyethylene. Polym. Test. 2005, 24, 71–80. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2002; ISBN 978-0-521-81681-6. [Google Scholar]
- Brown, P.S.; John, M.; Loadman, R.; Tinker, A.J. Applications of FT-NMR to Crosslink Density Determinations in Natural Rubber Blend Vulcanizates. Rubber Chem. Technol. 1992, 65, 744–760. [Google Scholar] [CrossRef]
- Bin Ahmad, A.; Bin Amu, A. Estimation of crosslink density by solid-state NMR spectroscopy. In Blends of Natural Rubber; Tinker, A.J., Jones, K.P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1998; pp. 40–52. ISBN 978-94-010-6064-6. [Google Scholar]
- Radusch, H.-J.; Kolesov, I.; Gohs, U.; Heinrich, G. Multiple Shape-Memory Behavior of Polyethylene/Polycyclooctene Blends Cross-Linked by Electron Irradiation. Macromol. Mater. Eng. 2012, 297, 1225–1234. [Google Scholar] [CrossRef]






| Weight % of BPO | Q | N/mol·m−3 | ΔfusH/J·g−1 | ΔcrystH/J·g−1 |
|---|---|---|---|---|
| 0 | 0 | 0 | 73.5 ± 2.1 | −67.6 ± 1.9 |
| 1 | 72.9 ± 2.6 | −64.3 ± 2.4 | ||
| 3 | 14.9 ± 0.5 | 48.4 ± 4 | 71.7 ± 1.8 | −65.2 ± 2.1 |
| 5 | 8.2 ± 0.1 | 143.3 ± 4 | 68.7 ± 2.4 | −65.2 ± 1.0 |
| 7 | 7.4 ± 0.2 | 177.6 ± 9 | 67.5 ± 2.0 | −60.6 ± 1.7 |
| 10 | 6.7 ± 0.04 | 209.2 ± 3 | 57.3 ± 1.2 | −49.2 ± 1.8 |
| Weight % of BPO | Cooling Rate/K·min−1 | Tonset/K | t1/2/s | n | Zc/min−n·K−1 |
|---|---|---|---|---|---|
| 0 | 10 | 307.2 | 27 | 2.05 | 1.13 |
| 0 | 20 | 303.6 | 13 | 1.96 | 1.14 |
| 1 | 10 | 306.1 | 33 | 2.04 | 1.09 |
| 1 | 20 | 302.3 | 22 | 1.96 | 1.08 |
| 3 | 10 | 304.9 | 33 | 2.01 | 1.09 |
| 3 | 20 | 301.3 | 22 | 1.96 | 1.08 |
| 5 | 10 | 303.6 | 45 | 2.12 | 1.02 |
| 5 | 20 | 299.9 | 29 | 2.01 | 1.06 |
| 7 | 10 | 302.5 | 50 | 2.39 | 1.01 |
| 7 | 20 | 298.6 | 29 | 2.33 | 1.07 |
| 10 | 10 | 293.0 | 37 | 2.24 | 1.07 |
| 10 | 20 | 289.2 | 23 | 2.21 | 1.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedov, I.; Magsumov, T.; Abdullin, A.; Yarko, E.; Mukhametzyanov, T.; Klimovitsky, A.; Schick, C. Influence of the Cross-Link Density on the Rate of Crystallization of Poly(ε-Caprolactone). Polymers 2018, 10, 902. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080902
Sedov I, Magsumov T, Abdullin A, Yarko E, Mukhametzyanov T, Klimovitsky A, Schick C. Influence of the Cross-Link Density on the Rate of Crystallization of Poly(ε-Caprolactone). Polymers. 2018; 10(8):902. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080902
Chicago/Turabian StyleSedov, Igor, Timur Magsumov, Albert Abdullin, Egor Yarko, Timur Mukhametzyanov, Alexander Klimovitsky, and Christoph Schick. 2018. "Influence of the Cross-Link Density on the Rate of Crystallization of Poly(ε-Caprolactone)" Polymers 10, no. 8: 902. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080902